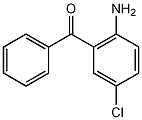
Structural formula
| Business number | 07F4 |
|---|---|
| Molecular formula | C13H10ClNO |
| Molecular weight | 231.68 |
| label |
2-Benzoyl-4-chloroaniline, 2-amino-5-chlorobenzophenone, 2-amino-5-chlorobenzophenone, 2-Amino-5-chlorobenzophenone, 4-Chloro-2-benzoylaniline, H2NC6H3(Cl)COC6H5, For the preparation of sedative drugs |
Numbering system
CAS number:719-59-5
MDL number:MFCD00007839
EINECS number:211-949-7
RTECS number:PC4933500
BRN number:475640
PubChem number:24890879
Physical property data
1. Character:Yellow or bright yellow needle crystal
2. Density (g/mL,25/4℃):1.33
3. Relative vapor density (g/mL,air=1): Undetermined
4. Melting point (ºC): 96 -100
5. Boiling point (ºC,5mmHg):207
6. Boiling point (ºC,5.2kPa): Undetermined
7. Refractive Index: Undetermined
8. Flashpoint (ºC): 211
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa,25ºC): Undetermined
12. Saturated vapor pressure (kPa,60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/Log value of partition coefficient (water): undetermined
17. Explosion limit (%,V/V): Undetermined
18. Lower explosion limit (%,V/V): Undetermined
19. Solubility: insoluble in water
Toxicological data
Acute toxicity: miceceliacLD50:681mg/ kg
Ecological data
Slightly hazardous to water. Do not allow undiluted or large quantities of product to come into contact with groundwater, waterways or sewage systems. Do not discharge material into the surrounding environment without government permission
Molecular structure data
1. Molar refractive index: 65.17
2. Molar volume(m3/mol):181.7
3. isotonic ratio(90.2K):488.6
4. Surface Tension(dyne/cm):52.2
5. Dielectric constant:
6. Dipole moment(10-24cm3):
7. Polarizability: 25.83
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.8
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 4
6. Topological molecule polar surface area 43.1
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 250
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure, avoid contact with oxides
Storage method
Keep container tightly sealed Take it out of the container and store it in a cool, dry place
Synthesis method
(1) is produced by the reaction of p-chloroaniline and benzoyl chloride. First, add p-chlorobenzene into the glass-lined reaction pot. -bidi-font-family: Tahoma; mso-font-kerning: 1.0pt; mso-ansi-language: EN-US; mso-fareast-language: ZH-CN; mso-bidi-language: AR-SA”>70 ℃The following, add anhydrous zinc chloride, add benzoyl chloride dropwise while stirring, then raise the temperature, and wait until the temperature is 195-205℃ Insulation2hAfter, use90-95℃ Wash five times with hot water (the water layer and washing liquid recover benzoic acid and zinc chloride) in100℃Slowly add the sulfuric acid at 142℃Insulation40min. Pour the liquid into the liquid while it is hot0-5℃In water, a solid precipitates. Under stirring, adjust with liquid sodapHNo higher than1, in20 -25℃Filter. The filtrate recovers p-chloroaniline. The filter cake is suspended in water and neutralized topH=6, filter dry, wash with water until neutral, and dry to obtain crude product. Then add 6-7ethanol,6%Activated carbon, reflux30min, filter and crystallize, bake Work hard and get quality products. (2) cyclization of p-nitrochlorobenzene and cyanobenzyl to obtain isoxazole, then ring opening and reduction And get.
Purpose
Used as a pharmaceutical intermediate, mainly used in the preparation of sedative drugs such as chlordiazepoxide, diazepam, diazepam, and sulfazepam



 微信扫一扫打赏
微信扫一扫打赏
