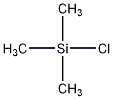
Structural formula
| Business number | 01K2 |
|---|---|
| Molecular formula | C3H9ClSi |
| Molecular weight | 108.64 |
| label |
Trimethylsilane chloride, Trimethylmonochlorosilane, Monochlorotrimethylsilane, Trimethylsilyl chloride, Chlorotrimethylsilane, TMSCl, Dow Corning® Z-1224, TMCS, Trimethylchlorosilane, Trimethylsilyl chloride, Cholrotrimethylsilane, Elemental organic compounds |
Numbering system
CAS number:75-77-4
MDL number:MFCD00000502
EINECS number:200-900-5
RTECS number:VV2710000
BRN number:1209232
PubChem number:24892952
Physical property data
1. Properties: colorless to light yellow transparent liquid with pungent odor. [18]
2. Melting point (℃): -57.7[19]
3. Boiling point (℃): 57[20]
4. Relative density (water = 1): 0.85[21]
5. Relative vapor Density (air=1): 3.8[22]
6. Saturated vapor pressure (kPa): 26.7 (20℃)[23]
7. Critical pressure (MPa): 3.36[24]
8. Octanol/water partition coefficient: 2.48[25] sup>
9. Flash point (℃): -18 (OC) [26]
10. Ignition temperature (℃): 395[27]
11. Explosion upper limit (%): 6[28]
12. Explosion lower limit (%): 1.8[29]
13. Solubility: soluble in benzene, methanol, ether, and perchlorethylene. [30]
Toxicological data
1. Skin/eye irritation
Standard Draize test: rabbit, skin contact: 500μL; severity of reaction: moderate.
Standard Draize test: Rabbit, eye contact: 5 μL; severity of reaction: moderate.
2. Acute toxicity: Oral LD50 in rats: 5660μL/kg; Inhaled LCLo in mice: 100mg/m3; Intraperitoneal LCLo in mice: 750mg/kg; Rabbit skin contact LD50: 1780μL/kg;
3. Chronic toxicity/carcinogenicity mice Intraperitoneal TCLo: 1000mg/kg/I;
4. Mutagenic microbial mice Salmonella typhi mutation: 1mg/plate;
5. Acute toxicity [31] LD50: 5660μl (4811mg)/kg (rat oral) ; 1780μl (1513mg)/kg (rabbit transdermal)
6. Irritation [32]
Rabbit transdermal Peel: 500μl, moderate irritation.
Rabbit eye: 5μl, moderate irritation.
7. Mutagenicity [33] Microbial mutagenicity: Salmonella typhimurium 1mg/dish.
Ecological data
Slightly hazardous to water, avoid contact of undiluted or large quantities of product with groundwater, waterways or sewage systems.
Molecular structure data
1. Molar refractive index: 29.51
2. Molar volume (cm3/mol): 125.1
3. Isotonic specific volume (90.2K ): 249.1
4. Surface tension (dyne/cm): 15.6
5. Polarizability (10-24cm3): 11.70 p>
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 28.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[34] Stable
2. Incompatible substances[35] Strong acid, strong alkali, water
3. Conditions to avoid contact[36] Humid air
4. Polymerization hazard[37] No polymerization
5. Decomposition products[38] Hydrogen chloride
Storage method
Storage Precautions[39] Store in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C and the container should be kept sealed. They should be stored separately from acids, alkalis, etc., and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Methyl chloride and silicon powder are directly synthesized in the next step catalyzed by cuprous chloride to generate a methylchlorosilane mixture, which can be purified by distillation to obtain trimethylchlorosilane and other monomers. Laboratory preparation can be made by reacting tetramethylsilane with acetyl chloride in the presence of aluminum trichloride.
2. Stir crude (or industrial product) trimethylchlorosilane with aluminum trichloride, aluminum tribromide or ferric hydroxide at 60°C for 10 minutes, and then distill to obtain pure product.
3. Methyl chloride and silicon powder are synthesized in one step at high temperatures above 300 to 550°C in the presence of cuprous chloride catalyst. The resulting methylchlorosilane mixture is purified by distillation to obtain trimethylchlorosilane.
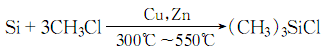
4. Connect the 17.5g quad Methylsilane was quickly added to 26.5g of purified aluminum trichloride cooled in an ice-salt bath. While stirring, 16ml of acetyl chloride was added dropwise.
The dripping speed of acetyl chloride is preferably to maintain appropriate reflux. The dripping process is completed in 40 minutes. After the dripping is completed, the reaction is stirred for 1 hour and 20.8g of trimethylchlorosilane can be obtained by distillation. The response is:
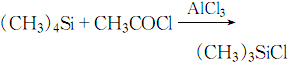
Purpose
1. Used as an intermediate, hydrophobic agent, and analytical reagent in the manufacture of silicone oil.
2. Used as a gas chromatography derivatization reagent for the silanization of unhindered hydroxyl, amino and carboxyl groups. Also used in organic synthesis.
3. Silanization reagents for hydroxyl, amino and carbonyl groups. Used to prepare its volatile derivatives for gas chromatography analysis. Ketol condensation of esters, condensation cyclization of α and ω monodioic acid esters, and acylation of malonate esters. Isosimilar acid esters are prepared from urethane. Preparation of enol silane ethers from carbonyl compounds. Preparation of enamines from ketones. Reductive silylation of aromatic rings, etc.
4. Trimethylchlorosilane is mostly used to synthesize silicon ether compounds and vinyl silane. It can also be used as a protective group for hydroxyl-containing compounds such as alcohols. In addition, it is also used in the synthesis of tert-butoxycarbonyl ( BOC) and other deprotection reactions.
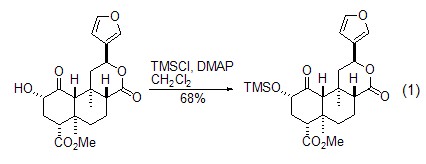
As a protective group An important application of trimethylchlorosilane is as alcohols[1] and phenols[2] , terminal alkyne [3,4], etc., react to form compounds containing trimethylsilyl groups. In the reaction with alcohol compounds, TMSCl generates silicon ether compounds under the action of bases such as triethylamine, DMAP, etc. This method can be used to protect the alcoholic hydroxyl groups in primary, secondary, and tertiary alcohols (Formula 1)[ 1].
Under similar conditions, TMSCl also It can react with ketone compounds to generate enol ether compounds (formula 2)[5~7]. Trimethylsilyl is easily removed under the action of acid.
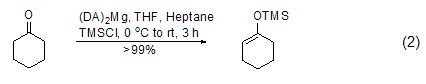
For terminal alkynes, in Under the action of lithium, zinc reagents, etc., terminal alkynes can directly interact with TMSCl to generate silane compounds (formula 3)[3,4].
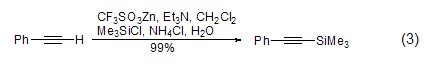
Under the action of strong alkali, TMSCl It is also possible to introduce a trimethylsilyl group (formula 4)[8] on the aromatic ring.
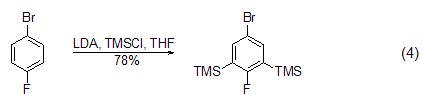
Addition reaction With the participation of transition metal catalysts or triphenylphosphine, etc., epoxy compounds can directly react with TMSCl The reaction is ring-opening, and the product is an O-end-protected silicon ether compound. After removing the silicon group, an alcohol compound (formula 5) [9,10] can be obtained.
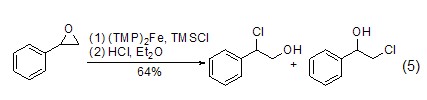
TMSCl can also be used with α,β-unsaturated carbonyl compounds undergo 1,4-conjugate addition reaction (Formula 6)[11~14]. p>
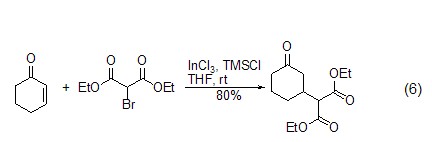
Elimination reaction In the presence of TMSCl and a catalyst, epoxides or allyl alcohol derivatives can undergo deoxygenation reactions to generate carbon-carbon double bond compounds (formula 7)[15,16]
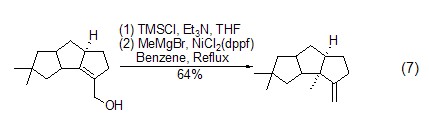
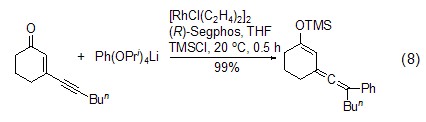
Formation of silyl vinyl accumulated dienes Under the action of transition metal catalysts, alkenes and alkynes can couple with TMSCl to generate accumulated dienes, which can be further oxidized to α, β -Unsaturated ketone (Formula 8)[17]
5. Used as an intermediate, hydrophobic agent, and analytical reagent in the manufacture of silicone oil. [40]
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/59.jpgextended-reading:https://www.bdmaee.net/dabco-2039-catalyst-cas3033-62-3-evonik-germany/extended-reading:https://www.newtopchem.com/archives/44177extended-reading:https://www.newtopchem.com/archives/45022extended-reading:https://www.bdmaee.net/n-methylimidazole/extended-reading:https://www.bdmaee.net/fascat8201-catalyst/extended-reading:https://www.bdmaee.net/toyocat-trc-catalyst-tosoh/extended-reading:https://www.newtopchem.com/archives/44661extended-reading:https://www.morpholine.org/4-acryloylmorpholine/extended-reading:https://www.bdmaee.net/dimethyltin-dichloride/



 微信扫一扫打赏
微信扫一扫打赏
