Background and overview[1]
Hexaaminobenzene (3-hydrochloride) is an aromatic hydrocarbon derivative that can be used as an intermediate in organic synthesis.
Preparation[1]
Simple synthesis of hexaaminobenzene (3 hydrochloride):
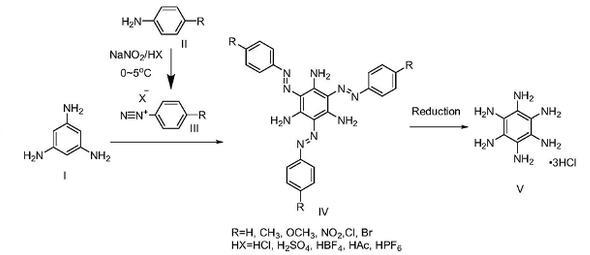
Step 1: Synthesis of 4-methylaniline diazonium salt of fluoroborate
Dissolve sodium nitrite (704mg, 1.01mmol, 1.01eq) in 3ml deionized water, place it in an ice bath to pre-cool for 10min; mix 50wt% tetrafluoroboric acid aqueous solution (3ml) and deionized water (3ml) After uniformity, place it in an ice-water mixture to pre-cool, add a certain amount of 4-methylaniline (1.074g, 10mmol, 1eq), mix the mixture evenly by magnetic stirring, then add 3ml NaNO aqueous solution dropwise, and react under magnetic stirring for 10 minutes. After the reaction was completed, the white needle-like solid was obtained by filtration, washed with ice water and dried to obtain 4-methylaniline diazonium salt of fluoroborate (1.8722g, yield 90.9%).
H-NMR (400MHz, DMSO) δ (ppm): 8.53 (d, J = 8.8Hz, 2H), 7.78 (d, J = 8.8Hz, 2H), 2.57 (s, 3H) ).
Step 2: Synthesis of 2,4,6-trisazo-p-tolyl-1,3,5-triaminobenzene
Dissolve 1,3,5-triaminobenzene (246.0mg, 2mmol, 1eq) in 20ml anhydrous methanol, and dissolve and disperse 4-methyldiazonium fluoroborate (1.65g, 8mmol, 4eq) in in 10ml anhydrous methanol. Drop 10ml of the methanol solution of 1,3,5-triaminobenzene into the methanol solution of 4-methylaniline diazonium salt of fluoroborate under magnetic stirring. After the dropwise addition is completed, react under magnetic stirring for 20 minutes. The reaction temperature is 20℃. After the reaction, the orange flocculent precipitate was obtained by filtration, washed with methanol and dried to obtain compound 2,4,6-trisazo-p-tolyl-1,3,5-triaminobenzene (614.4 mg, 1.29 mmol, yield 64.4%).
H-NMR (400MHz, DMSO-d6) δ (ppm): 9.48 (s, 6H), 7.78 (d, J = 8.4Hz, 6H), 7.30 (d, J = 8.4Hz) , 6H), 2.37 (s, 9H).
Step 3: Synthesis of hexaaminobenzene (3 hydrochloride)
Completely dissolve tin dichloride (4.848g, 21mmol, 21eq) in concentrated hydrochloric acid (7ml, 37wt%, 84mmol), and then add 2,4,6-trisazo-p-tolyl-1,3,5 – Triaminobenzene (477.4 mg, 1 mmol, 1 eq) was dispersed in ethyl acetate (20 ml). Add the ethyl acetate dispersion dropwise to the concentrated hydrochloric acid solution of SnCl stirred on a magnetic stirrer, and heat and reflux at 90°C for 2 hours to ensure complete reaction. A light pink precipitate appears in the system, and the color of the liquid changes from orange to close to colorless. After suction filtration, the solid was washed with 10 ml of ethyl acetate, 10 ml of methanol, and 10 ml of diethyl ether, and dried to obtain hexaaminobenzene (3 hydrochloride) powdery solid (205.2 mg, yield 74.0%).
Main reference materials
[1] CN201810476387.6 A synthesis method of hexaaminobenzene hydrochloride



 微信扫一扫打赏
微信扫一扫打赏
