Background and overview[1]
p-Nitrophenyl-β-D-glucuropyranoside is a sugar derivative and can be used as a pharmaceutical intermediate.
Preparation[1]
The specific steps for preparing p-nitrophenyl-β-D-glucuropyranoside are as follows:
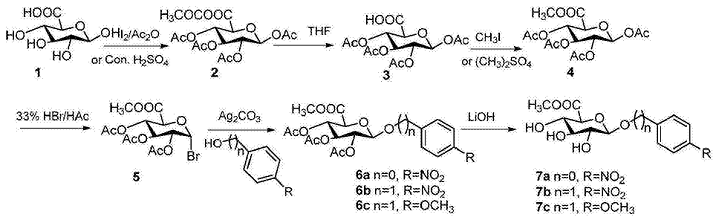
1) Preparation of 1,2,3,4-tetrakis-O-acetylglucuronic acid acetic anhydride (2)
Under nitrogen protection, 20 grams of β-D-glucuronic acid (1) (103.0mmol) was dissolved in 100mL of acetic anhydride, 8 drops of concentrated sulfuric acid was added dropwise, the reaction temperature was raised to 60°C, and the reaction was continued for 1 hour. to room temperature, concentrate under reduced pressure, evaporate acetic anhydride to 1/4, add diethyl ether, a white solid precipitates, and dry under vacuum to obtain 39.3g of pentaacetylglucuronic anhydride with a yield of 94.3%. Alternatively, add a catalytic amount of iodine instead of concentrated sulfuric acid, stir and react at 40°C for 2 hours, and use the same post-treatment to obtain 38.4g of pentaacetyl glucuronate with a yield of 92.1%. 1HNMR (400MHz, DMSO-d6) δ: 5.99 (d, J=8Hz, 1H), 5.46 (t, J=12, 8Hz, 1H), 5.04 (t, J=12, 8Hz, 1H), 4.94 (t , J=12, 8Hz, 1H), 4.51 (d, J=12Hz, 1H), 2.07 (s, 3H), 2.0 (s, 3H), 1.97 (s, 3H), 1.96 (s, 3H), 1.90 (s, 3H).13CNMR (100MHz, DMSO-d6) δ: 172.46, 169.88, 169.50, 169.10, 168.36, 91.01, 72.02, 71.58, 70.26, 69.13, 21.48, 20.88, 20.75, 20.72, 20.70.
2) Preparation of 1,2,3,4-tetrakis-O-acetyl-D-glucuronic acid (3)
Under nitrogen protection, 21g of 1,2,3,4-Tetra-O-acetyl-glucuronic acid acetic anhydride (2) (51.94mmoL) was dissolved in 150mL of a mixed solution of tetrahydrofuran and water (1:1=V /V), stir overnight, evaporate tetrahydrofuran under reduced pressure, filter, and dry under vacuum to obtain 18.0g white solid, yield 95.7%. 1HNMR (400MHz, DMSO-d6) δ: 13.47 (brs, 1H), 5.99 (d, J) =8Hz, 1H), 5.46 (t, J = 12, 8Hz, 1H), 5.05 (t, J = 12, 8Hz, 1H), 4.95 (t, J = 12, 8Hz, 1H), 4.51 (d, J =8Hz, 1H), 2.07 (s, 3H), 2.00 (s, 3H), 1.97 (s, 3H), 1.96 (s, 3H).13CNMR (100MHz, DMSO-d6) δ: 169.88, 169.50, 169.17, 168.35, 91.01, 72.01, 71.58, 70.26, 69.13, 20.88, 20.75, 20.71, 20.70.
3) Preparation of 1,2,3,4-tetrakis-O-acetyl-D-glucuronate methyl ester (4)
Under nitrogen protection, 19.2g tetraacetyl-O-glucuronic acid (3) (52.99mmol) was dissolved in 200 ml of dry N,N-dimethylformamide, 29.0g potassium carbonate (204.32mmol) and Slowly add 12.0g methyl iodide (84.48mmoL), continue stirring for 2 hours, pour into 800g ice water, stir vigorously, filter, wash with distilled water, and vacuum dry the filter cake to obtain 19.2g of white solid tetraacetyl-O-methyl glucuronate. , yield 96.4%. 1HNMR (400MHz, CDCl3) δ: 5.77 (d, J=8Hz, 1H), 5.30 (t, J=12, 8Hz, 1H), 5.23 (t, J=12, 8Hz, 1H), 5.13 (t, J =12, 8Hz, 1H), 4.18 (d, J = 8Hz, 1H), 3, 74 (s, 3H), 2.11 (s, 3H), 2.03 (s, 3H), 2.02 (s, 3H).13CNMR (100MHz, CDCl3) δ: 169.88, 169.38, 169.15, 168.80, 166.76, 91.31, 72.94, 71.77, 70.09, 68.87, 53.00, 20.75, 20.54, 20.51, 20.44.
4) Preparation of 2,3,4-tri-O-acetyl-α-D-bromoglucuronate methyl ester (5)
Under nitrogen protection, dissolve 25.6 grams of 1,2,3,4-tetra-O-acetyl-β-D-glucuronate methyl ester (4) (68.0mmol) into 120 ml of dichloromethane , cool to 0°C, add dropwise 150mL of 33% HBr acetic acid solution, continue stirring at this temperature for 2 hours, monitor by TLC, when completed, add water to dilute, extract with dichloromethane, and wash the organic layer with saturated sodium bicarbonate and saturated brine. , dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and purified the crude product by silica gel column chromatography. The mobile phase was petroleum ether: ethyl acetate (4::1, V/V) to obtain 22.7 grams of white solid, with a yield of 83.9 %. 1HNMR (400MHz, CDCl3) δ: 6.51 (d, J=4Hz, 1H), 5.44 (t, J=12, 8Hz, 1H), 5.09 (t, J=12, 8Hz, 1H), 4.73 (t, J =12, 8Hz, 1H), 4.42 (d, J = 12Hz, 1H), 3, 61 (s, 3H), 1.95 (s, 3H), 1.91 (s, 3H), 1.90 (s, 3H).13CNMR (100MHz, CDCl3) δ: 169.40, 169.38, 169.20, 166.44, 85.54, 71.89, 70.06, 69.09, 68.24, 52.90, 20.37, 20.22.
5) Preparation of p-nitrophenyl-2,3,4-tri-O-acetyl-β-D-glucuronate methyl ester glycoside (6a)
Under nitrogen protection, 20.8 grams of silver carbonate (75.6mmoL), 5.3 grams (37.8mmoL) of p-nitrophenol, and a catalytic amount of iodine (0.3g) were respectively dissolved in 40 mL of methylene chloride, added with molecular sieves, and stirred for 10 mins. Dissolve 10 grams of 2,3,4-tri-O-acetyl α-D bromoglucuronate (5) (25.2mmoL) and 10 ml of methylene chloride and slowly add it. After the dripping is completed, wrap it with tin foil. Reaction 24 hours, add ethyl acetate to dilute, filter through diatomaceous earth, concentrate the filtrate under reduced pressure, and purify the crude product on a silica gel column. Use petroleum ether and ethyl acetate (5:1, V/V) to prepare 10.0 g of white solid, with a yield of 88.1%. 1HNMR (400MHz, CDCl3) δ: 8.18 (m, 2H), 7.07 (m, 2H), 5.38-5.28 (m, 4H), 4.26 (m, 1H), 3.71 (s, 3H), 2.06 (s, 3H ), 2.05 (s, 3H), 2.04 (s, 3H). 13CNMR (100MHz, CDCl3) δ: 169.99, 169.34, 169.13, 166.58, 161.02, 143.29, 125.81, 116.58, 98.05, 72.57, 70.56, 68.69, 20 .58, 20, 59, 20.47.
6) Preparation of p-nitrobenzyl-β-D-glucuronide (7a)
In a nitrogen environment, 17.8 grams of p-nitrophenyl 2,3,4-tri-O-acetyl-β-D-glucuronate methyl ester glycoside (6a) (40mmol) solution was added to anhydrous methanol solution , add 4.8 grams of lithium hydroxide (200mmoL), stir at room temperature for 4 secondsAt this time, TLC monitors the process. Dowex50WX resin is added to neutralize excess alkali, filtered, concentrated to obtain p-nitrophenyl-β-D-glucuronide, and recrystallized with ethanol to obtain 10.7 grams of white flaky solid, yield 85 %.
1HNMR (400MHz, D2O) δ: 8.15 (m, 2H), 7.14 (m, 2H), 5.20 (d, J = 8Hz, 1H), 4.06 (t, J = 12Hz, 1H), 3.59 (dd , J=8, 6.0Hz, 1H), 3.56 (d, J=12.0Hz, 6.0Hz, 1H), 3.55 (dd, J=8.0, 6.0Hz, 1H).
Main reference materials
[1] CN201810503213.4 Preparation method of substituted benzyl or substituted phenyl β-D-hexuronic acid glycoside



 微信扫一扫打赏
微信扫一扫打赏
