Background and overview[1]
Dimethyl isophthalate is a white needle-like crystal, soluble in methanol and ethanol, but insoluble in water. It is used in organic synthesis and as a fixed plate for gas chromatography. Dimethyl isophthalate is obtained by the esterification of isophthalic acid and methanol.
Preparation[2-3]
Report 1,
Add 70Kg isophthalic acid and 0.6Kg N, N-dimethylformamide into the synthesis tank, then add 105Kg thionyl chloride, start to slowly raise the temperature, and control the reaction temperature to maintain reflux at 50-80°C. At the same time, the vacuum is controlled to 90-97MPa. The exhaust gas first passes through the water absorption system, which absorbs the hydrogen chloride into more than 30% hydrochloric acid, and then passes through the alkali absorption system to convert the sulfur dioxide into the by-product sodium sulfite. After the synthesis reaction proceeds for 5-7 hours, excess unreacted thionyl chloride is recovered. Control the flow rate to 7-10Kg/h, slowly add this crude synthetic solution to the reaction kettle containing 40Kg of methanol, keep the reaction temperature at 60-90°C, and control the vacuum at 90-95MPa. The exhaust gas passes through the water absorption system, which absorbs hydrogen chloride into more than 30% hydrochloric acid. After the dropwise addition is completed, the reaction is continued for 5-10 hours, and excess unreacted methanol is recovered. The synthetic liquid prepared above is rectified, and the vacuum degree is controlled to be less than 1.3KPa. After removing the previous fraction, collect the fraction at 115-125°C to obtain 76Kg of dimethyl isophthalate, with a content of 99.94%.
Report 2,
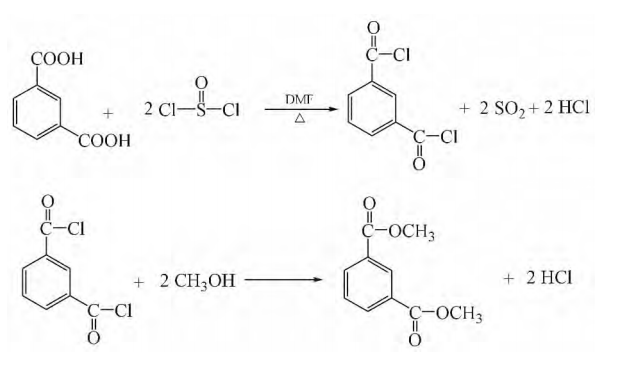
Weigh 0.30 mol of isophthalic acid and add it to a 500 mL three-necked bottle containing 0.84 mol of thionyl chloride. After adding 0.45 g of DMF under stirring, reflux at a pressure of 93300~97320 Pa and 50~80°C. Reaction, the hydrogen chloride in the tail gas is absorbed by water, and the sulfur dioxide is absorbed by saturated sodium carbonate solution. Wait until the reaction liquid changes from a milky white turbid liquid to a light yellow clear liquid, then heat the reaction for 0.5 hours, recover excess thionyl chloride under a negative pressure of 90660~96000Pa, and obtain a light yellow isophthaloyl chloride solution. At 50°C and a certain negative pressure, gradually add the above synthesized isophthaloyl chloride into a stirred 500mL three-necked flask containing excess methanol. After the addition is completed, continue to heat to 60-80°C for reflux reaction. After 0.5h until no bubbles are generated, excess methanol is recovered under a negative pressure of 90660~96000Pa to obtain a light yellow solution. Use a distillation device instead, perform distillation under a negative pressure of 666~1333Pa, collect the fraction between 120~125℃, and obtain a colorless, clear and transparent liquid. After cooling, it becomes 53g white crystals, with a yield of 91%. Purity (GC) 99.92%; m.p.67.5~68.2℃; IR (KBr, v/cm-1): 2960, 1728, 1440, 1290, 1249, 726; 1HNMR (500MHz, CDCl3), δ: 3.95 (s, 6H), 7.53 (t, J=7.8Hz, 1H), 8.22 (d, J=7.8Hz, 2H), 8.68 (s, 1H).
Apply[4]
Wang Honglong and others used isosorbide and dimethyl isophthalate to modify conventional PET polyester. The modifier addition amount was 7% (mole fraction), and IS-PET and DI-PET were prepared. , IS-DI-PET three kinds of modified polyester. Nuclear magnetic resonance showed that the modifying groups were introduced into the polyester macromolecular chain to achieve the purpose of modification. Comparative analysis of the modified polyester and conventional PET showed that DSC showed that the Tg temperature of IS-PET and IS-DI-PET polyester increased and the melting point decreased. TGA showed that the modified polyester still had good thermal stability. The tensile properties and standard moisture regain of three types of polyesters were tested after spinning. The results showed that when the isosorbide and isophenylene structures are modified individually, they can enhance the fiber strength, but when modified together, the fiber strength is reduced; When sorbitol is modified alone, the fiber hygroscopicity is significantly improved.
References
[1] Concise Dictionary of Fine Chemicals
[2][China Invention] CN201210540192.6 Method for synthesizing dimethyl isophthalate
[3] Ma Haibing, Chen Yongjun, Zhu Chuantao, Zhu Xiaona. Synthesis of high-purity dimethyl isophthalate [J]. Fine Chemical Intermediates, 2014, 44(01): 31-33.
[4] Wang Honglong, Tian Xiuzhi, Wang Shugen, Jiang Xue. Preparation and properties of isosorbide-dimethyl isophthalate modified polyester [J]. Journal of Wuhan Engineering Vocational and Technical College, 2015, 27(04 ):8-11.



 微信扫一扫打赏
微信扫一扫打赏
