Background and overview[1][2]
2-Methylsulfonylbenzeneboronic acid is an important organic synthesis intermediate. It can be used to synthesize derivatives of polyolefins, styrene and biphenyl through suzuki aryl coupling reaction, and can be used in many natural products and organic materials. in the process of synthesis.
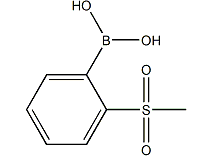
2-methylsulfonylbenzeneboronic acid
Preparation[2,3]
Add 1800kg of water, 700kg of sulfone sulfonyl chloride, 450kg of sodium bicarbonate and 330kg of sodium sulfite into the salt-forming kettle, and carry out the salt-forming reaction under normal pressure. The reaction temperature is 60°C and the holding time is 4 hours. The carbon dioxide gas produced by the reaction and Empty a small amount of water, and after the reaction is completed, filter it while it is hot at 40-50°C. The mother liquor is pumped into the methylation kettle by vacuum, and the filter residue is processed separately. The mother liquor is further heated to 70°C in the methylation kettle, and 158kg of methyl chloride gas is passed under a pressure of 0.5kg/cm3, and the mixture is kept warm and cooled for crystallization. When the crystallization mother liquor is cooled to 40°C, the material is discharged and filtered, and the filter cake is centrifuged by a centrifuge. The filtered mother liquor and the centrifuged mother liquor are concentrated and distilled. The water vapor is condensed in the condenser and then used, and the waste salt generated is processed separately. After the wet product is dried in a desiccator, methylsulfonylbenzene is obtained. The content of methylsulfonylbenzene is above 99%, and the yield is about 70%. Bromination of methylsulfonylbenzene gives o-bromomethylsulfonylbenzene.
Add 0.05mol (0.1eq) o-bromomethylsulfonylbenzene, 0.55mol (1.1eq) magnesium chips, a few grains of elemental iodine, and 300ml THF into the reaction bottle, stir, raise the temperature to reflux, initiate Grignard initiation, and continue in the reflux state Add 0.45 mol (0.9 eq) of m-bromotoluene dropwise. After the dropwise addition is completed, reflux the heat preservation reaction. When the reaction time is up, cool down to -60.0°C and start to dropwise add 1.0 mol (2.0eq) of trimethyl borate. After the dropwise addition is completed, keep the reaction until the raw materials have reacted. Raise the temperature, add water, and add hydrochloric acid dropwise. Hydrolyze, adjust pH to 1-2, insulate for reaction, when the reaction time is up, rotary evaporate, drain, beat and purify, and dry to obtain white solid 2-methylsulfonylbenzeneboronic acid 0.44 mol, moisture 0.05%, purity 98.64%, yield is 88.0%.
Main reference materials
[1] Deng Jiyong, & Lin Yuanbin. (2005). Synthesis of 4,6-dimethoxyi-2-methylsulfonyl-pyrimidine%4, 6-dimethoxy-2-methylsulfonylpyrimidine. Fine Chemical Intermediates, 035(003), 9-10.
[2] Zhu Zhenghua, Cheng Zhusheng, Duan Caiming, Yu Ronggen, & Hao Zhimin. (1986). Synthesis of 2-methylsulfonyl pyrimidine compounds and their use in the preparation of reactive dyes. Journal of East China Institute of Chemical Technology (06), 22- 30.
[3] Cheng Sixi, & Du Shenghua. (2009). Synthesis of 2-methylsulfonyl-5-trifluoromethyl-1,3,4-thiadiazole. Pesticides, 48(4), 247-248.



 微信扫一扫打赏
微信扫一扫打赏
