Overview[1]
Resorcinol is an ether organic compound that can be used as a pharmaceutical synthesis intermediate.
Apply[1-3]
1) Synthesis of 2,4-dimethoxyethylbenzene from isophthalic ether. 2,4-Dimethoxyethylbenzene is an important aromatic ether chemical intermediate with multiple uses. It is mainly used to synthesize the benzamide antipsychotic drug etapride and can also be used to synthesize chlorinated aromatic ethers. It is a soil pest insecticide, and its research has broad application prospects.
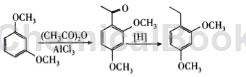
In a 100mL three-necked flask, add 5.1g of acetic anhydride (about 0.05mol) and 60mL of chloroform in sequence, and add 13.50g of anhydrous AlCl3 (about 0.101mol) while stirring in an ice bath. After the addition is complete, continue stirring in an ice bath until the temperature drops to 0°C. Start dropping 10 mL of chloroform solution containing 6.90 g (0.05 mol) of isophthalic ether. Keep the temperature at no more than 5°C during the dropwise addition. After the addition, remove the ice bath and stir the reaction at 35°C for 5 hours. After the reaction is completed, add a mixture of ice and water to the reaction bottle under an ice bath (control the temperature in the bottle to be below 10°C) to decompose the aluminum trichloride, then separate the organic phase and wash it with water three times, and then dry it with anhydrous sodium sulfate. The solid was filtered off and the solvent was evaporated to dryness under reduced pressure, then dissolved in 90% ethanol and left to crystallize at 0°C. Finally, 7.04g of yellow crystals were obtained, with a yield of 78.2% and a bp of 38 to 40°C.
2) Preparation of 2,2′-dihydroxy-4,4′-dimethoxybenzophenone. The method is: 1. Prepare isolylene dimethyl ether and oxalyl chloride in the presence of a catalyst at 70- The intermediate product 2,2’4,4′-tetramethoxybenzophenone was obtained by the reaction at 80°C. The ratio of the amounts of isolylene dimethyl ether and oxalyl chloride was 1:1-20. The used The catalyst is azoisobutyronitrile or benzoyl peroxide, and the dosage is 0.5%-2% of the mass of isophthalic ether; 2. React the obtained intermediate product with Lewis acid under the condition of organic reagent as solvent. The temperature is 50°C and the time is 2-3 hours. After stopping the reaction, add water for hydrolysis, liquid separation, rotary evaporation and recrystallization to obtain 2,2′-dihydroxy-4,4′-dimethoxybenzophenone. The Lewis acid is one of AlCl3, ZnCl2, BF3, and polyphosphoric acid; the organic reagent is one of dichloroethane, toluene, xylene, nitrobenzene, and chlorobenzene. The reaction temperature of this method is suitable, the catalyst is simple and easy to obtain, the dosage is small, the environmental pollution is small, and it is closer to the requirements of green chemistry.
3) Preparing 2,6-dimethoxybenzoic acid, which includes the following steps: 1. Add metallic sodium to toluene and heat and melt it to make sodium sand; 2. Add sodium sand to the bottle at 0°C After standing for a period of time, add chlorobenzene when the temperature rises to 22~25°C; add a catalyst, put sodium sand and chlorobenzene into toluene solvent and react to form sodium phenyl; thirdly, sodium phenyl is left for a period of time, at a temperature of Add isophthalic ether at 25~27℃, and sodium phenyl reacts with isophthalic ether to produce 2,6-dimethoxyphenyl sodium; four, the 2,6-dimethoxyphenyl sodium produced by the reaction in three Sodium phenyl reacts with carbon dioxide below 0°C to produce sodium 2,6-dimethoxybenzoate; five, the product obtained in step four is subjected to acid precipitation to obtain crude 2,6-dimethoxybenzoic acid; six, Crystallize the crude 2,6-dimethoxybenzoic acid product through a methanol/water system to obtain the 2,6-dimethoxybenzoic acid product, which is then dried.
Preparation
isophenylenedimethyl ether is prepared as follows:
①Put resorcinol and 20%-50% sodium hydroxide aqueous solution into the reaction tank, and dissolve to obtain a mixed solution; the molar ratio of resorcinol to sodium hydroxide aqueous solution is 1:1-1.2 ;
② Heat the mixture in step ① to 30℃-45℃, then add dimethyl sulfate, and at the same time add 20%-50% sodium hydroxide aqueous solution dropwise, and keep the reaction for 2-4 hours. The molar ratio to resorcinol is 2.4-3:1, and the amount of 20%-50% sodium hydroxide aqueous solution dropped is the same as the 20%-50% sodium hydroxide aqueous solution in step ①;
③ Separate the solution obtained after the insulation reaction in step ② is complete, and use a distillation tower to distill the organic phase obtained after separation to obtain isolylene dimethyl ether.
Main reference materials
[1] Synthesis of 2,4-dimethoxyethylbenzene from isophthalic ether
[2] CN201310443361.9 A preparation method of 2,2′-dihydroxy-4,4′-dimethoxybenzophenone
[3] CN200810024327.7 A kind of 2,6-dioic acidSynthetic method of oxybenzoic acid



 微信扫一扫打赏
微信扫一扫打赏
