Overview[1][2]
O-carboxybenzaldehyde has the properties of aldehyde and acid. It can form ester with alcohol, reduce Ag(NH3)2NO3, form oxime with H2NOH, and can form anhydride (diphenylphthalide) when heated to the melting point. Preparation method: obtained by hydrolysis of o-dichloromethylbenzoyl chloride in the presence of CaCO3. Uses: Used as reagents for organic synthesis.
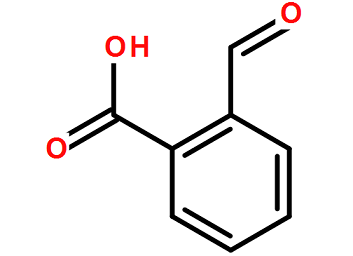
Preparation[1]
A synthesis method of o-carboxybenzaldehyde, including the following steps:
(1) Take 20mL o-xylene and place it in the reaction kettle, heat the reaction kettle to 132°C, and add the solvent N, N-dimethylformamide DMF, catalyst and cocatalyst in sequence, the N, The amount of N-dimethylformamide DMF added was 22mL, the amount of catalyst and cocatalyst were 2.5g and 12.5g respectively, the oxygen input amount was controlled to 900mL/min, the hydrogen input amount was 100mL/min, and the reaction was carried out for 4 hours. ;
(2) Cool the above reaction solution to room temperature, and the transparent colorless crystals obtained are o-tolyl benzonitrile. After washing the obtained crystals twice with DMF, filter out the DMF solvent to obtain high purity Based on 90% o-toluonitrile;
(3) Place the o-toluenenitrile synthesized in the above step (2) into the reaction kettle, heat the reaction kettle to 113°C, and add the solvent N, N-dimethylformamide DMF and the catalyst in sequence and cocatalyst, the addition amount of N, N-dimethylformamide DMF is 13mL, the addition amount of catalyst and cocatalyst are 1.2g and 8.5g respectively, the oxygen introduction amount is controlled to 900mL/min, and the hydrogen introduction The amount is 100mL/min, react for 2 hours, and the white crystalline powder obtained is 4-cyanobenzaldehyde;
(4) Add 32 mL of solvent to dissolve the 4-cyanobenzaldehyde synthesized above, then add sulfuric acid to adjust to pH=5. After hydrolysis, o-carboxybenzaldehyde can be obtained.
Apply[3-4]
CN201510027695.7 discloses a method for synthesizing butylphthalide. Butylphthalide is the first national first-class anti-cerebral ischemia new drug with completely independent intellectual property rights and a new chemical structure after my country joined the WHO. It is also the world’s first new anti-cerebral ischemia drug. The first new chemical drug with “ischemic stroke treatment” as its main indication. It uses o-carboxybenzaldehyde as a starting material, reacts with n-butylmagnesium chloride Grignard reagent with THF as a solvent, and adjusts the acid to prepare a butylphthalide product; it also involves a process for preparing high-purity butylphthalide. The obtained crude butylphthalide is hydrolyzed with an alkaline substance, and then the solid is precipitated by adjusting acid and filtered to obtain the butylphthalide intermediate; repeat the above process of adjusting acid and alkali, and finally perform ring closing and desolvation under reduced pressure to obtain high purity of butylphthalide. The synthesis method of the present invention avoids the use of low flash point ether as a solvent, the purification process is simple to operate, the reagents used are easy to purchase in large quantities, and does not require column chromatography to purify products and vacuum distillation under high temperature and high vacuum, and is easy to scale up industrial production.
CN201210504410.0 discloses a fluorescent probe for detecting intracellular hydrogen sulfide and its preparation method and application. Cy7 fluorescent dye and sodium acetate are dissolved in anhydrous DMF, and heated and refluxed for 2-4 hours under Ar gas protection. , the obtained mixture is cooled and then filtered, the mother liquor is crystallized at a low temperature of -4~20°C, and the reddish-brown crystals of ketocyanine are obtained by suction filtration; dissolve o-carboxybenzaldehyde and oxalyl chloride in anhydrous CH2Cl2 and then add a small amount of DMF. Stir for 2-6 hours at 0°C to obtain o-formylbenzoyl chloride; dissolve ketocyanine and triethylamine in anhydrous CH2Cl2, cool to 0°C, add o-formylbenzoyl chloride dropwise and stir at 0°C For 10-30 minutes, the mixture was heated to room temperature, stirred overnight, filtered with suction, spun to dryness, separated with a chromatographic column, and spun to dryness to obtain a green solid with the following structural formula. The fluorescent probe of the present invention is simple to synthesize, has fast detection speed, does not require long-term incubation during the detection process, has high sensitivity, and can detect endogenous hydrogen sulfide in cells.
Main reference materials
[1] Compound Dictionary
[2] [Chinese invention] CN201810514546.7 A synthesis method of o-formyl benzoic acid
[3] Synthesis method and purification process of CN201510027695.7 butylphthalide
[4] CN201210504410.0 A fluorescent probe for detecting intracellular hydrogen sulfide and its preparation method and application



 微信扫一扫打赏
微信扫一扫打赏
