Background and overview[1]
Aromatic aldehydes are important organic chemical raw materials and intermediates and are widely used in the fields of medicine, dyes, pesticides, food additives, spices and flavors. With the increase in demand for flavors and fragrances in my country, the important role of aromatic aldehydes in fragrances has attracted much attention. tert-Butylbenzaldehyde is an important raw material for fine chemicals and electronic chemicals such as drugs, dyes, flavors and fragrances. It is in great demand especially in the synthesis of fragrances and flavors – lily aldehyde.
At present, the synthesis methods of p-tert-butylbenzaldehyde include chemical oxidation, oxygen (air) oxidation, electrochemical oxidation and benzyl halide hydrolysis. The oxygen (air) liquid phase oxidation method is currently the most interesting method for people. It does not consume expensive oxidants, has large production capacity, and has little environmental pollution. It is a method that currently has development potential. However, the catalyst synthesis process required by this method is not mature enough, and the reaction temperature is very high. Therefore, the thermal stability of the organic raw materials and oxidation products under the reaction conditions is very high. The oxidants used in the chemical oxidation method include Mn2O3, MnO2, H2O2 and organic peroxide (TBHP). This process has simple synthesis steps, high yield, and high product purity. It is favored in the synthesis process of lily aldehyde because it has no chlorine, pure aroma, and no impurities. Although there are shortcomings such as corrosion of equipment and a large amount of by-product MnSO4, the electrochemical oxidation method can oxidize MnSO4 into Mn2O3 and MnO2, which can be recycled, thereby reducing environmental pollution, lowering production costs, and making the synthesis process green.
Preparation method[1]
1. Chemical oxidation method
Chemical oxidation method refers to the use of oxidants other than air and oxygen to oxidize p-tert-butyltoluene. The oxidants used are MnO2, H2O2 and organic peroxide (TBHP). The traditional industrial production of p-tert-butylbenzaldehyde uses chemical oxidation method, that is, using MnO2 as the oxidant, and directly oxidizing p-tert-butyltoluene in H2SO4 to synthesize p-tert-butylbenzaldehyde. The chemical reaction formula is as follows:
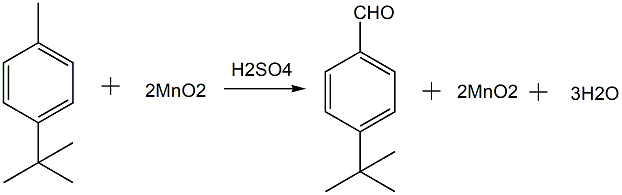
This process has simple steps, high p-tert-butylbenzaldehyde yield (can reach more than 90%), and high product purity. It is favored in the synthesis process of lily aldehyde because it has no chlorine, pure aroma and no impurities; The disadvantages are that this process has equipment corrosion, serious pollution, difficulty in recovering a large amount of MnSO4, high production costs, and difficulty in product separation. A relatively in-depth study was conducted on the liquid-phase homogeneous oxidation of tert-butyltoluene with hydrogen peroxide. At 45°C, a homogeneous Co2+-Br–H2O2 oxidation system is used to oxidize p-tert-butyltoluene. The conversion rate of p-tert-butyltoluene is 25% to 30%, and the selectivity of p-tert-butylbenzaldehyde is 80%. Ce3+-Br–HAc-H2O2 oxidation system was used to oxidize p-tert-butyltoluene. When the reaction temperature was 70°C, a conversion rate of 41% and a selectivity of 59% were obtained. Most experimental results showed poor selectivity. H2O2 is a green and environmentally friendly oxidant. It turns into water after the reaction, has no harmful residues, and the reaction conditions are mild. However, in the above-mentioned homogeneous catalytic system, there are also shortcomings such as complicated product separation.
2. Oxygen (air) oxidation method
The oxygen (air) oxidation method includes liquid phase oxidation reaction and gas-solid phase oxidation reaction. The oxygen (air) liquid phase oxidation method is currently the most interesting method, and there are many patents for preparing aromatic aldehydes using this method. Under the reaction conditions of 150℃ and 5.6MPa, dioxane is used as the solvent, Co-Mn-Br (1mmolCo(OAc)2·4H2O, 3mmolMn(OAc)2·4H2O, 10mmolNaBr) is used as the catalyst, liquid phase O2 Oxidation of p-tert-butyltoluene (33.58mmol), the conversion rate of p-tert-butyltoluene was 16.4%, and the selectivity of p-tert-butylbenzaldehyde was 87%. However, in the above-mentioned Co-Mn-Br ternary composite catalyst liquid phase oxidation of p-tert-butyltoluene system, Br- severely corrodes expensive titanium equipment, and the CH3Br(g) produced destroys the ozone layer, and there are also shortcomings such as difficulty in product separation.
3. Electrolytic oxidation method
There are many studies on the preparation of p-tert-butylbenzaldehyde by electrolytic oxidation of p-tert-butyltoluene. In a non-diaphragm polyethylene plastic electrolytic cell, carbon rods are used as anode and cathode, and a mixture of methanol, acetic acid and sodium fluoroborate is used as electrolyte to electrolyze p-tert-butyltoluene and obtain 40% selectivity of p-tert-butylbenzaldehyde. . The German BASF Company has studied the electrochemical oxidation of toluene to prepare p-tert-butylbenzaldehyde, the raw material for the synthesis of lily aldehyde, but the electrolytic oxidation method was not used in actual production. The electrolytic oxidation of p-tert-butyltoluene to prepare p-tert-butylbenzaldehyde requires a large amount of circulating water treatment. The trace presence of aldehyde in the water phase will lead to a serious decline in current efficiency and high power consumption; and the electrodes and electrolytic cells Material issues such as membranes and separators have not been well resolved, thus limiting the industrial application of this method.
4. Other synthesis methods
Synthesis of p-tert-butylbenzaldehyde using benzyl chloride hydrolysis method. p-tert-butyl benzyl chloride is prepared from p-tert-butyltoluene under the action of sulfuric acid, formaldehyde and concentrated hydrochloric acid. p-tert-butyl benzyl chloride reacts with hexamethylenetetramine in dilute acetic acid and hydrolyzes to obtain the product. The process conditions of this method are mature, but the reaction steps are many and the yield is low; the product contains trace amounts of organic chlorines that are difficult to remove and are carcinogenic; and a large amount of sodium chloride or calcium chloride wastewater is produced in the subsequent treatment section. Cause serious pollution to the environment.
Main reference informationMaterial
[1] Synthesis of p-tert-butylbenzaldehyde
[2] Research progress on the synthesis of p-tert-butylbenzaldehyde



 微信扫一扫打赏
微信扫一扫打赏
