Overview[1]
4-Nitrotoluene-2-sulfonic acid autocondensation dyes can be divided into dyes with colors ranging from yellow to red according to the degree of condensation. Among them, the most widely used direct yellow 11 dye is a strong yellow dye, especially suitable for Dyeing of fibers, leather, etc. As described in U.S. Patent 4310331, the production process is mainly as follows: 4-nitrotoluene-2-sulfonic acid is catalyzed by alkali metal hydroxides, especially sodium hydroxide, at 50-90°C for half an hour to self-polycondensation reaction. After several hours, sulfuric acid or hydrochloric acid is used to neutralize excess alkali, and finally the direct yellow 11 wet cake is obtained through press filtration.
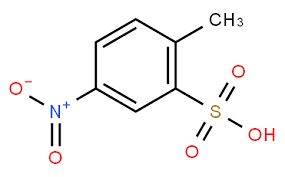
US3905949 uses the lithium salt of 4-nitrotoluene-2-sulfonic acid as a raw material and condenses it in the presence of lithium hydroxide to obtain a highly concentrated paste dye that is easily soluble in water. The lithium salts of the azo- and azo-stilbene oxide dyes thus obtained are not only easily soluble in water but also have greater substantivity than the sodium salts. However, lithium salts also have the disadvantage that they do not form stable dye solutions; instead, their concentrated solutions have a tendency to gel, crystallize or clump during storage, making the dye difficult or even impossible to use.
Apply[3]
Preparation of 4,4′-dinitrostilbene-2,2′-disulfonic acid
At 40°C, put a mixture of 400ml water, 210g methanol, 200g ethylene glycol dimethyl ether, 0.5g magnesium sulfate (II) and 90g sodium hydroxide into a 2-liter glass reactor. While vigorously introducing gas into the solution at a rate of 40 liters of air per hour at atmospheric pressure, a total of 510g of 30.5% 4-nitrotoluene-2-sulfonic acid in 72% (by weight) water and One-fourth of the solution of 28% (weight) ethylene glycol dimethyl ether in the mixed solvent and added in 10 minutes. Then it took 15 minutes to raise the temperature to 50°C. The remaining 4-nitro-2-sulfonic acid solution was metered in. The addition was completed in 1 hour. At the same time, 110g of sodium hydroxide solution with a concentration of 27.3% was added. After the metered addition is completed, let the mixture continue to react at 50°C for a total of 5.5 hours. 30 minutes after the metered addition is completed, the air flow is reduced to 20 1/h, and after another 2 hours, it is reduced to 10 1/h. After the reaction is complete, use 80% sulfuric acid to neutralize excess alkali. HPLC analysis showed that the yield of 4,4′-dinitrostilbene-2,2′-disulfonate disodium was 90.4% of the theoretical value.
The preparation method of dye direct yellow 11 is as follows:
Slowly add 11.9 parts of 4-nitrotoluene-2-sulfonic acid to 44 parts of 25% tetramethylammonium hydroxide aqueous solution (40°C), and stir to form a blue-purple solution. Stir the reaction for 5 hours at 70°C. After the reaction is completed, a reddish-brown transparent solution is formed. 4.7 parts of 30% hydrochloric acid are added to the solution to adjust the pH to 8 to obtain a yellow solution with a maximum absorption wavelength of 414 nm.
The structural formula of the dye Direct Orange 15 is as follows:
Slowly add 12.2 parts of 4-nitrotoluene-2-sulfonic acid to 40 parts of 25% tetramethylammonium hydroxide aqueous solution (40°C), stir evenly to form a blue-purple solution. Heat to 65°C and stir for 6 hours. After the reaction, a reddish-brown transparent solution was formed. After cooling to 50°C, add 2 parts of glucose, stir and react for 0.5 h, lower the solution to room temperature, and then add 2.9 parts of 30% hydrochloric acid. Add 150 parts of water to the solution to dilute it to obtain a clear orange solution with a maximum absorption wavelength of 440nm.
Main reference materials
[1] Gao Wentao. . Preliminary study on the reaction mechanism of preparing 4,4,’-dinitrostilbene-2,2′-disulfonic acid by oxygen liquid phase catalytic oxidation of 4-nitrotoluene-2-sulfonic acid %primary mechanism investigation of catalytic oxidation of 4- nitrotoluene- 2 – sulfonic acid to 4,4′ – dinitrostilbene- 2,2′-disulfonic acid. Journal of Bohai University (Natural Science Edition), 023(3), 1-9.
[2] Gao Wentao, & Zhang Shufen. . Study on the liquid-phase catalytic oxidation of p-nitrotoluene orthosulfonic acid with oxygen. Journal of Liaoning University of Engineering and Technology: Natural Science Edition, 18(4), 434-436.
[3] Huang Xiaobo, Zhang Junke, Cai Chun, Liao Weiqiu, Lu Chunxu, & HUANGXiao-bo et al. (2000). Synthesis process of 4,4′-dinitrostilbene-2,2′-disulfonic acid Improvement. Fine Chemicals, 17(3), 175-177.



 微信扫一扫打赏
微信扫一扫打赏
