Background and overview[1]
4-Iodobenzenesulfonyl chloride is an aromatic hydrocarbon derivative and can be used as a pharmaceutical synthesis intermediate.
Preparation method[1]
4-Iodobenzenesulfonyl chloride is prepared as follows: Into a dry 5 L three-neck round bottom flask equipped with mechanical stirring, a heating mantle, a water-cooled condenser, a connecting hose of the condenser and a 2 L addition funnel and an inert Add 2.5 L of chloroform and chlorosulfonic acid (503 g, 4.32 mol) to the gas inlet together. The mixture was heated to gentle reflux and a solution of iodobenzene (400 g; 1.96 mol) in 0.5 L chloroform was added over 1 h, during which time the color of the reaction mixture changed from yellow to deep red-purple with HCl of release. The reaction was heated at reflux for an additional hour. Analysis by GC showed complete conversion to p-iodobenzenesulfonyl chloride. The reaction was worked up by pouring the mixture into a 6 L separatory funnel and discarding the lower, mostly inorganic acid layer. The organic layer was neutralized and dried over MgSO4. The solvent was evaporated to obtain 4-iodobenzenesulfonyl chloride as a crude yellow solid; the yield was approximately 593 g (quantitative). If desired, the product can be further purified by distillation under high vacuum.
Apply[2]
4-iodobenzenesulfonyl chloride can be used as a pharmaceutical synthesis intermediate. For example, when preparing benzenesulfonylamino)-L-asparagine (8-2), the following reaction occurs:
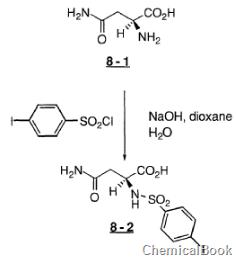
To a stirred solution of acid 8-1 (4.39g, 33.2mmol), NaOH (1.49g, 37.2mmol), dioxane (30ml) and H2O (30ml), 4-iodobenzenesulfonate was added Acid chloride (10.34g, 34.2mmol). After about 5 minutes, NaOH (1.49, 37.2 mmol) dissolved in 15 ml H2O was added and the cooling bath was removed. After 2.0 hours, the reaction mixture was concentrated. The residue was dissolved in H2O (300 ml) and washed with EtOAc. The water portion was cooled to 0°C and then acidified with concentrated hydrochloric acid. The solid was collected and washed with Et2O to give acid 8-2 as a white solid.
1HNMR (300MHz, D2O) δ7.86 (d, 2H, J=8HZ), 7.48 (d, 2H, J=8Hz) 3.70 (m, 1H), 2.39 (m, 2H).
Main reference materials
[1] US5602185 – SUBSTITUTED TRIFLUOROSTYRENE COMPOSITIONS
[2] WO1998046220 – COMBINATION THERAPY FOR THE PREVENTION AND TREATMENT OF OSTEOPOROSIS



 微信扫一扫打赏
微信扫一扫打赏
