Background and overview[1]
2-Amino-3-(2-chlorobenzoyl)-5-ethylthiophene can be used as a pharmaceutical synthesis intermediate. If 2-amino-3-(2-chlorobenzoyl)-5-ethylthiophene is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing and rinse thoroughly with soap and water. If you feel discomfort on your skin, seek medical attention; if it comes into contact with your eyes, separate your eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse your mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1]
2-Amino-3-(2-chlorobenzoyl)-5-ethylthiophene can be used as a pharmaceutical synthesis intermediate. If it is used as a reactant, the following reaction occurs:
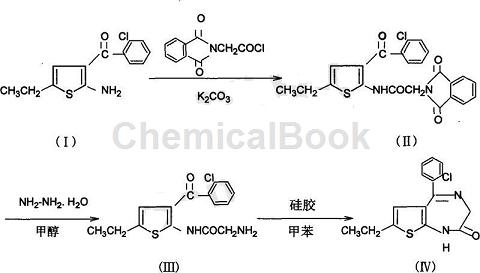
1) Synthesis of 2-phthalylglycinamide-3-o-chlorobenzoyl-5-ethylthiophene (II)
Dissolve 120g (97%) 2-amino-3-(2-chlorobenzoyl)-5-ethylthiophene (I) and 105g (98%) phthalylglycyl chloride in 600ml Add 90g anhydrous potassium carbonate to the dioxane under stirring, stir at room temperature for 4 hours, filter the precipitate, wash it once with a small amount of dioxane, then wash it with water until neutral, and dry to obtain 161g of white powdery solid (II) (HPLC97%), mp196~8°C. After concentrating the dioxane filtrate, 18.4g of yellow solid (II) (HPLC 96.5%) was obtained, mp was 195°C, and the total yield was 90.43%.
2) Synthesis of 2-aminoacetamide-3-o-chlorobenzoyl-5-ethylthiophene (III)
Mix 50g (97%) (II) and 250ml methanol, stir and heat to 50°C, then add 7g (80%) hydrazine hydrate, heat and reflux for one hour, recover methanol under reduced pressure, and extract the residue with 100ml ethyl acetate , filter out the insoluble matter (phthaloyl hydrazide), and wash once with an appropriate amount of ethyl acetate. Combine the ethyl acetate solutions, wash once with water, dry with anhydrous magnesium sulfate to recover the ethyl acetate, dissolve the residue with 20 ml of toluene, decolorize, crystallize at room temperature, filter to obtain light yellow crystals 2-aminoacetamide-3-o-chlorobenzyl Acyl-5-ethylthiophene (III) 21g (HPLC 98.61%) (consistent with the standard), yield 60.8%. 2g of crystals were recovered from the mother liquor (HPLC), which contained 87.3% of (III) and 1.49% of (IV).
3) 5-O-chlorophenyl-7-ethyl-1,3-dihydro-3-H-thieno[1,3-e][1,4]diaza-2-one ( IV) synthesis
Mix 100g (97%) 2-phthalylglycinamide-3-o-chlorobenzoyl-5-ethylthiophene (II) and 500ml methanol, stir and heat to 50°C, then add 14g ( 80%) hydrazine hydrate, stir and reflux for 1 hour, cool to room temperature, filter out the product phthaloyl hydrazide, wash once with a small amount of methanol, combine the methanol solution, and dilute it with about 4 to 5 times of water to obtain a yellow-green viscous substance (III, IV mixture), pour off the methanol diluent, add 500ml toluene and 200g silica gel, reflux and stir for dehydration in a three-necked flask equipped with a water separator for 5 hours, evaporate the toluene under reduced pressure, and wash with (500ml×3) ethanol Silica gel, combine the ethanol solutions, recover the ethanol under reduced pressure, dissolve the residue in 15 ml of toluene, and freeze crystallize to obtain yellow crystals 5-o-chlorophenyl-7-ethyl-1,2-dihydro-3H-thieno[ 1,3-e][1,4]aza-2-one, mp202~204℃ (HPLC98.38%), the filtrate was concentrated and crystallized with a small amount of ethyl acetate to obtain 7g (IV) (HPLC98.4%) , mp202~204℃, the total yield is 66.68%.
Main reference materials
[1] Synthesis method of CN200910195170.92-aminoacetamide-3-o-chlorobenzoyl-5-ethylthiophene



 微信扫一扫打赏
微信扫一扫打赏
