Overview[1][2]
4-Methyl-2-phenyl-1,3-thiazole-5-carbonyl chloride is a heterocyclic organic compound that can be used as a pharmaceutical synthesis intermediate.
Preparation[1]
1) Synthesis of benzoyl chloride
Add 3.35mmol benzoic acid and 1.373g (11.5mmol) thionyl chloride into a 50mL eggplant-shaped flask, stir under magnetic stirring and reflux for 2 hours, evaporate the unreacted thionyl chloride under reduced pressure, and then add petroleum ether (2× 1 mL), continued distillation under reduced pressure, and after cooling to room temperature, was used directly in the next reaction without further purification.
2) Synthesis of benzamide
Add 40 mL of 25% ammonia water into a 100 mL eggplant-shaped flask, bring it to 5°C in an ice-water bath, add dropwise a theoretical amount of 66.1 mmol benzoyl chloride CH2Cl2 solution while stirring, continue stirring for 1 hour after addition, precipitate a solid, and concentrate under reduced pressure. Filter and wash with water to obtain the product, 9.752g of white solid. The filtrate is extracted with ethyl acetate and dried over anhydrous sodium sulfate.
3) Synthesis of phenylsulfonamide
Add 3.02mmol benzamide (3), 0.222g (1.00mmol) phosphorus pentasulfide, and 15mL dry THF into a 50mL eggplant-shaped flask, stir under magnetic stirring and reflux. TLC monitored the reaction. After refluxing for 4 hours, the raw material points basically disappeared and the reaction was stopped. Cool to room temperature, concentrate under reduced pressure, add saturated Na2CO3 aqueous solution to adjust pH to 6, add 15 mL of ethyl acetate, remove impurities by suction filtration, separate the filtrate layers, extract the aqueous phase with ethyl acetate (2×5 mL), combine the organic phases, and remove the impurities. Dry with water Na2SO4, filter and concentrate to obtain pure product, 0.392g of yellow solid, yield 77.8%.
4) Synthesis of 4-methyl-2-phenyl-1,3-thiazole-5-carbonyl chloride
Add 25.7mmol phenylsulfamide (4) and 100mL absolute ethanol into a 250mL three-neck flask, add 4.348g (26.4mmol) ethyl 2-chloroacetoacetate dropwise while stirring, complete the dropwise addition, and heat to reflux. TLC monitored the reaction. After refluxing for 4 hours, the raw material points basically disappeared and the reaction was stopped. Cool to room temperature, concentrate under reduced pressure, wash the product with diethyl ether to obtain a crude product, acid chloride, and recrystallize from 80% ethanol to obtain the product.
Apply[1]
4-Methyl-2-phenyl-1,3-thiazole-5-carbonyl chloride can be used as a pharmaceutical synthesis intermediate. For example, prepare the following compounds:
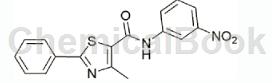
Add 0.696g (5.04mmol) m-nitroaniline, 15mL methylene chloride and 1mL triethylamine into a 50mL three-neck flask, bring the ice water bath to 5°C, and add dropwise 1.195g (5.03mmol) 4-methyl under stirring. A solution of base-2-phenyl-1,3-thiazole-5-carbonyl chloride in dichloromethane was stirred at room temperature. TLC monitored the reaction. After stirring for 2 hours, the raw material points basically disappeared and the reaction was stopped. Filter and concentrate to obtain a crude product, which is recrystallized from dichloromethane to obtain 0.724g of yellow solid.
Main reference materials
[1](CN102532123) Thiazole-5-carboxamide compound, its preparation method, pharmaceutical composition and use



 微信扫一扫打赏
微信扫一扫打赏
