Background and overview[1][3]
Trihexyphenidyl, chemical name (±)-α-cyclohexyl-α-phenyl-1-piperidine propanol, molecular formula: C20H31NO, molecular weight: 301.47, trihexyphenidyl is used as an anti-Parkinson’s drug Disease drugs were launched abroad in the 1950s and began to be launched domestically in the 1960s. This product is a central anticholinergic and anti-Parkinson’s disease drug. It is used to treat tremor symptoms in the elderly. Its function is to selectively block the cholinergic nerve pathway of the striatum, but has little peripheral effect, thus helping to restore Parkinson’s disease. The balance of dopamine and acetylcholine in the brain of patients with the disease improves the symptoms of Parkinson’s disease. It is clinically used for paralysis tremens, post-encephalitis or paralysis tremor caused by arteriosclerosis. It is effective in improving salivation. It is less effective in relieving stiffness and bradykinesia. It improves tremor significantly, but the overall effect is not as good as levodopa and amantadine. It is used for patients with mild symptoms and those who cannot tolerate levodopa. It is often used in combination with levodopa. Hepatolenticular degeneration. dystonia dystonia, epilepsy, chronic schizophrenia, akathisia due to antipsychotic drugs. It can also be used for extrapyramidal disorders caused by drugs.
Trihexyphenidyl is clinically used to treat shaking paralysis, shaking paralysis caused by encephalitis or arteriosclerosis. It is effective in improving salivation and hepatolenticular degeneration, Parkinson’s disease, encephalitis or arteriosclerosis. Parkinson’s disease, dystonia and other diseases. However, excessive use of such drugs can cause side effects such as dry mouth, constipation, urinary retention, mydriasis, blurred vision, brain failure and liver damage caused by drug sensitivity. Therefore, they are classified as controlled drugs in most countries in the world.
Preparation of oxidized impurities[1]
Trihexyphenidyl oxidation impurities are of great significance to the in-depth study of trihexyphenidyl. The literature Journal of Pharmaceutical Sciences.1995,84(5):561-567 reports the preparation process of trihexyphenidyl oxidation impurities such as the compound represented by formula I. : In tetrahydrofuran solvent, m-chloroperoxybenzoic acid was used as the oxidant, and the reaction was carried out at -70°C for 0.5 hours, with a yield of 52%:
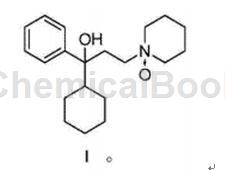
CN201910376883.9 provides a method for preparing the compound of formula I and provides a qualified reference substance for the quality control of trihexyphenidyl. The technical solution of the present invention is: a method for preparing a compound of formula I, which includes the following steps: oxidizing the reactant trihexyphenidyl or its salt in an organic solvent under the action of an oxidizing agent to obtain a compound of formula I.
Example:
Take 0.5g of trihexyphenidyl hydrochloride solid (1.48mmol, 1.0eq), add it to 5ml of methylene chloride, stir, and add 0.3g of m-chloroperoxybenzoic acid (1.73mmol, 1.17eq) at 20-25°C. , stir for 2 hours at 20-25°C. There is a new point in the TLC monitoring reaction. Add silica gel, concentrate to dryness under reduced pressure, put it on a silica gel column, and perform purification and separation. The eluent is petroleum ether/ethyl acetate = 15/1 ~ 10/1. Concentrate to obtain 0.2g of product, which is collected. The rate is 42%. MS(m/z):318.1[M+H]+. 1H NMR(400MHz, CDCl3)δ:7.42-7.43(d,2H), 7.28-7.32(t,2H),7.17-7.20(t,1H),3.45-3.48(m,1H),3.19-3.24(m ,1H),3.03-3.13(m,2H),2.87-2.98(m,2H)2.63-2.69(m,1H),2.17-2.31(m,3H),1.95-1.98(m,1H),1.49- 1.77(m,7H), 1.01-1.36(m,8H).
Application[2]
CN201710000246.2 provides a Chinese and Western medicine composite preparation for treating hypertension-induced stroke and a preparation method. The raw materials for making the active ingredients of the Chinese and Western medicine composite preparation are: Dian Changshan, Shanzhigen, Zeqin, Baogai Grass, half maple lotus, Chinese fir leaf, beech root, cypress, black sesame, longan meat, mountain ginger, red ginseng, phenobarbital, profenamine, trihexyphenidyl and prazosin; the traditional Chinese medicine and prazosin of the present invention The combination of Western medicine and Western medicine has the functions of activating blood circulation, relaxing muscles and activating blood circulation, dispelling wind and removing dampness, warming the middle and removing blood stasis, opening and closing obstruction, etc. It can be combined with the blood pressure-lowering effects of Azerbaijan, Shanzhigen, and phenobarbital. The present invention is more suitable for preventing and treating stroke induced by hypertension, can provide comprehensive treatment for stroke induced by hypertension, has significant curative effect, solves the shortcomings of existing Western medicine and traditional Chinese medicine, and has good clinical application value.
Detection method[3]
A method for preparing a trihexyphenidyl molecularly imprinted electrochemical sensor, including the following steps:
Use water as the solvent to prepare 20 mL of a mixed aqueous solution of cobalt sulfate with a concentration of 0.25 mol/L, nickel sulfate with a concentration of 0.25 mol/L and vitamin C with a concentration of 0.5 mol/L. Use benzyl alcohol as the solvent to prepare the concentration. 0.05 mol/L pentaerythritol distearyl diphosphite 5 mL, and then add the prepared 5 mL pentaerythritol distearyl diphosphite solution dropwise to the 20 mL cobalt sulfate prepared above at a rate of 5 drops/second. , nickel sulfate and vitamin C mixed solution, stir vigorously at 2500 rpm during the dropwise addition, and then react in a 50 mL hot water kettle at 130°C for 2 hours. After cooling, centrifuge to collect the resulting precipitate. Wash three times with water and ethanol, collect the precipitate by centrifugation, and vacuum dry the collected precipitate at 50°C to obtain a cobalt-nickel layered powder composite material;
To 10.0 mL solvent ethanol, add 1.5 mmol ~ 5.0 mmol template molecule trihexyphenidyl, 2.5 mmol ~ 7.5 mmol functional monomer abscisic acid, 4.5 mmol cross-linking agent oleuropein, and 0.15 mmol initiator. Azobisisobutyronitrile, 0.0100 g to 0.0300 g of cobalt-nickel layered powder composite material prepared in step and 0.015 g of 4-ethoxy-4′-cyanobiphenyl sensing film modifier ,���Add a chemical reagent and dissolve it with ultrasonic waves for 8 minutes;
Take 8 μL of the mixture from step and apply it on the surface of a clean and smooth glassy carbon electrode with a diameter of 2 mm. After leaving it for 6 hours, place the modified electrode in a vacuum drying oven at 85°C and heat it. Polymerize for 1.5 hours, and then use a mixed solvent of acetic acid and isobutanol with an eluent molar ratio of 3:1 to elute the template molecules to obtain.
Main reference materials
[1] [Chinese invention] CN201910376883.9 Preparation method of trihexyphenidyl oxidation impurity
[2] CN201710000246.2 A composite preparation of Chinese and Western medicine for treating hypertension-induced stroke and its preparation method
[3] [Chinese invention] CN201811309613.8 Preparation method of trihexyphenidyl molecularly imprinted electrochemical sensor



 微信扫一扫打赏
微信扫一扫打赏
