Overview[1]
Tetrakis(4-aminophenyl)methane is also called 4,4′,4″,4″′-methane tetraphenylamine, which can be prepared by the reduction of tetrakis(4-nitrophenyl)methane. It can be used as an intermediate for synthetic materials.
Preparation[1]
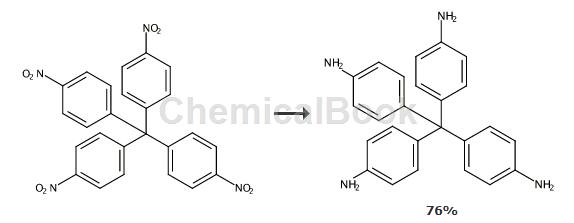
To a 250mL round-bottomed flask equipped with a magnetic stirrer and an oil bath, add tetrakis(4-nitrophenyl)methane (6.50g, 13.00mmol), Pd/C (0.98g, 15% by weight) and n-butyl Alcohol (150mL). With nitrogen bubbled through, the mixture was stirred and heated at 50°C for 15 minutes. Hydrazine hydrate (7.6 mL, 156 mmol) was added dropwise via the addition funnel while maintaining the temperature at 50-60°C. The dropwise addition was completed within 30 minutes. Subsequently, the reaction was stirred at 50°C for an additional 2 hours. The reaction remained heterogeneous throughout time. HPLC analysis showed that all starting material had been consumed and the product appeared as the only component. After cooling to room temperature, the mixture was purged with nitrogen for 10 minutes, then filtered and rinsed with THF. The solvent was removed by rotary evaporation and the residue was crystallized from ethanol to obtain 3.76 g (76%) of light-colored crystalline product. MS and NMR results are consistent with the proposed structure.
Apply[1]
Can be used to prepare N, N′, N″, N″′-(4,4′,4″,4″′-methanetetrakis(benzene-4,1-diyl))tetrakis(2,4, 5-trimethyl-N- (2,4,5-trimethylphenyl)aniline), used in electronic devices.
In a dry box operating glove box, add 4,4′,4″,4″′-methanetetraphenylamine (1.00g, 3.08mmol), 1-bromo-2,4,5-trimethylbenzene ( 1.35 g, 3.67 mmol), tris(tert-butyl)phosphine (0.10 g, 0.50 mmol) and Pd2(DBA)3 (0.23 g, 0.25 mmol) were mixed into a round-bottomed flask and dissolved in 120 mL of anhydrous toluene. The solution was stirred for one minute and then sodium tert-butoxide (2.72 g, 28.33 mmol) and 5 mL of anhydrous toluene were added. A heating mantle was added and the reaction was heated to 80°C over 18 hours. More 1-bromo-2,4,5-trimethylbenzene (350 mg) and NaOtBu (180 mg) were added and the reaction was stirred at 80°C for an additional 24 hours. The reaction mixture was then cooled to room temperature and filtered, washing with toluene (200 mL). The solvent was removed by rotary evaporation and the residue was redissolved in chloroform (40 mL). The crude product was precipitated in methanol (300 mL) and collected by filtration (3.3 g, 85% purity). The crude product was further purified by silica gel column chromatography using a gradient elution of chloroform in hexanes (5-21%). Recrystallization from chloroform and ethanol gave 2.60 g (64%) of the product as a pale yellow solid.
Main reference materials
[1] [Invented in China] CN201080046786.0 Triarylamine compounds for electronic applications



 微信扫一扫打赏
微信扫一扫打赏
