Background and overview[1]
Eltrombopag (trade name: Promacta) was developed by the British company GlaxoSmithKline. It was approved by the FDA for marketing in the United States in November 2008. It is an oral platelet production factor drug and a small molecule platelet production agent. It is a hormone receptor agonist that can interact with the thrombopoietin receptor in the transmembrane region of the human body to produce a signal cascade amplification, thereby inducing the proliferation and differentiation of bone marrow megakaryocytes. It is mainly used to treat thrombocytopenia in patients with chronic idiopathic thrombocytopenic purpura (ITP) who have failed treatment with glucocorticoids and immunoglobulins or who have undergone splenectomy. The drug is currently being studied for the treatment of hepatitis C virus, thrombocytopenia caused by chronic liver disease, and tumor-related thrombocytopenia. Eltrombopag, chemical name is 3′-{(2Z)-2-[1-(3,4-xylyl)-3-methyl-5-oxo-1,5-dihydro -4H-pyrazole-4-ylidene]hydrazino}-2′-hydroxy-3-biphenylcarboxylic acid. From the structure of the compound, the synthesis of this compound contains two important intermediates: 2-hydroxyl -3′-nitro-biphenyl-3-carboxylic acid (BPCA) and 1-(3,4-dimethylphenyl)-3-methyl-1H-pyrazol-5H-4-one.
Preparation[1]
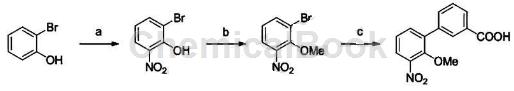
One of the preparation methods of 2-hydroxy-3′-nitro-biphenyl-3-carboxylic acid: add 10g N-(2-hydroxyphenyl) benzyl carbamate and 9g 3-bromobenzoic acid into a 250ml reaction bottle , 0.73gRhCl(cod)2, 1.28g triphenoxyphosphine, 40g cesium carbonate and 100ml toluene, reflux reaction under nitrogen protection, and TLC monitored the reaction to be complete. 100 ml of water was added, extracted with ethyl acetate, dried and concentrated to obtain the product (13 g, yield: 87%, HPLC purity: 97%). The above product is added to the hydrogenation kettle, 2g of 10% palladium on carbon (containing about 63% water) and 150ml of methanol are added, and hydrogen gas of 0.1-0.3MPa is introduced and stirred at room temperature. After the reaction is completed, filter and concentrate, add 100 ml of water to dissolve, adjust the pH to about 5.5 with 1M hydrochloric acid, precipitate the solid, filter it with suction, and dry to obtain a reddish-brown solid 2-hydroxy-3′-nitro-biphenyl-3-carboxylic acid ( 9g, yield: 95%, HPLC purity: 99%). 1HNMR (600MHz, DMSO-d6): δ6.50 (dd, J=1.8, 7.2Hz, 1H), 6.67~6.73 (m, 2H), 7.52 (t, J=7.8Hz, 1H), 7.71~7.73 ( m, 1H), 7.87 (dt, J = 1.2, 7.8Hz, 1H), 8.09 (t, J = 1.2Hz, 1H), MSm/z: 230.1 [M+H+].
Main reference materials
[1]CN201710815755.0 A synthesis method of eltrombopag intermediate



 微信扫一扫打赏
微信扫一扫打赏
