Background and overview[1]
2,4-Dichloro-6-fluorobenzaldehyde can be used as a pharmaceutical synthesis intermediate.
Preparation[1]
The preparation of 2,4-dichloro-6-fluorobenzaldehyde is as follows:

To a solution of 1,3-dichloro-5-fluorobenzene (2.0g, 12mmol) in tetrahydrofuran (20mL) at -70°C, lithium diisopropylamide (2M solution in tetrahydrofuran, 18mmol) was added dropwise. 9.1mL). The reaction was stirred at -70 °C for 0.5 h, then N,N-dimethylformamide (1.8 g, 24 mmol) was slowly added and stirring was continued at -70 °C for 0.5 h. The reaction was quenched with water (20 mL) and extracted with ethyl acetate (3 x 20 mL). The combined organic layers were concentrated in vacuo and purified by silica gel chromatography [petroleum ether: ethyl acetate = 30:1] to obtain 2,4-dichloro-6-fluorobenzaldehyde (1.5g, 64% yield), It is a yellow solid. 1H-NMR (CDCl3, 400MHz): 510.41 (s, 1H), 7.934 (s, 1H), 7.18-7.16 (d, J=10.0Hz, 1H).
Application
2,4-Dichloro-6-fluorobenzaldehyde can be used as a pharmaceutical synthesis intermediate. If the following reaction occurs:
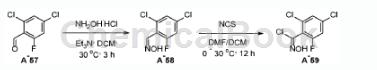
1) Dissolve 2,4-dichloro-6-fluorobenzaldehyde (1eq), hydroxylamine hydrochloride (1.3-2 eq.) and triethylamine (2eq.) in dichloromethane (1.2-2.5mL/mmol aldehyde ) was stirred at room temperature for 16 hours. Upon completion, the reaction mixture was diluted with water and extracted with dichloromethane (3 × 20 mL). The combined organic layers were washed with water and brine, dried over anhydrous sodium sulfate and concentrated in vacuo to give the oxime intermediate. The intermediate was purified by silica gel chromatography or used in the next step without further purification.
2) To a solution of the oxime intermediate (1 equiv) in dichloromethane (10 mL) at 0°C, add N-chlorosuccinimide (1.2 equiv) in N,N-dimethylformamide (0.5mL) solution. The mixture was stirred at 30°C for 1 hour. Upon completion, the reaction mixture was diluted with water and extracted with dichloromethane (3 × 10 mL). The combined organic layers were washed with water and brine, dried over anhydrous sodium sulfate, filtered and concentrated in vacuo to give the N-hydroxyimidyl chloride intermediate.
Main reference materials
[1](WO2016201096)AMINOBENZISOXAZOLECOMPOUNDSASAGONISTSOFA7-NICOTINICACETYLCHOLINERECEPTORS



 微信扫一扫打赏
微信扫一扫打赏
