Background and overview[1]
3-Nitrophenylmethanesulfonyl chloride is an organic intermediate that can be prepared from O-ethyl S-3-nitrobenzyl dithiocarbonate in two steps.
Preparation[1]
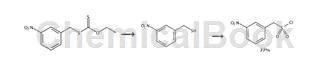
(3-Nitrophenyl)methanethiol. A solution of O-ethyl S-3-nitrobenzyl dithiocarbonate (11.55 g, 44.9 mmol) in DMSO (20 mL) was added to ethylenediamine (4.8 mL, 1.6 equiv) and concentrated HCl (1.8 mL, 0.5 equiv) in DMSO (20 mL) that had been allowed to cool to room temperature. The resulting mixture was stirred for 5 minutes, then diluted with 1M HCl (100 mL), then water (300 mL), and extracted with MTBE (200 mL). The organic layer was then extracted with 5% aqueous NaOH (3 × 50 mL) and the combined aqueous extracts were immediately acidified with 30 mL of 37% HCl. The mixture was extracted with dichloromethane (30 mL), and the combined organic phases were dried over MgSO4, stirred with 2 g of silica gel, filtered, and concentrated under reduced pressure. This product is light yellow oil. Yield 6.71g (39.7mmol, 88%). 1H NMR (CDCl3, 400MHz) δ1.87 (t, J = 7.9Hz, 1H), 3.84 (d, J = 7.9Hz, 2H), 7.51 (t, J = 7.9Hz, 1H), 7.66-7.69 (m, 1H), 8.11 (ddd, J = 8.2, 2.2, 1.0Hz, 1H), 8.21 (tt, J = 1.8, 0.4Hz, 1H). 13C NMR (CDCl3, 100MHz) δ28.3,122.1, 123.0,129.6,134.2,143.1,148.4. IR(NaCl) 3069,2031,1525,1351,810, 739,701,680, 667cm-1. EI-MS m/z 169 (56, [M]+), 136 (100, 121 (22), 90 (52), 78 (20), 63 (23).EI-HRMS m/ z Calculation [M ]+ C7H7NO2S 169.0197, found 169.0203.
Preparation of (3-nitrophenyl)methanesulfonyl chloride. Add m-nitrothiol (6.18 g, 36.5 mmol), methylene chloride (75 mL) and concentrated HCl (60 mL, 20 equiv) to the flask, then assemble the stir bar and reflux condenser. Then add hydrogen peroxide (30% aqueous solution, 22 mL, 6 equiv) from a pressure-equalizing addition funnel attached to the top of the condenser. After adding the first 5 mL of H2O2, stir the mixture rapidly without external heating until reflux begins. The remaining H2O2 was then added in 2 mL portions at a rate sufficient to maintain gentle reflux. After the mixture cooled, the green organic layer was separated, carefully decolorized (exothermically) with aqueous Na2SO3, dried with MgSO4, and Concentrate under reduced pressure. The residue was recrystallized from benzene-cyclohexane. The product is colorless crystal. Yield 6.60g (28.0mmol, 77%). Melting point 101-102℃ (Lit.8 95-100℃). 1H NMR (CDCl3, 400MHz) δ4.96 (s, 2H), 7.69 (td, J = 7.9, 0.7Hz, 1H), 7.85 (dt, J = 7.3, 1.6Hz, 1H), 8.36 (dd, J = 2.2, 1.0Hz, 1H), 8.37-8.39 (m, 1H). 13C NMR (CDCl3, 100MHz) δ69.3, 125.1, 126.2, 128.2, 130.4, 137.2, 148.5. IR (NaCl) 3082,2992,2930,1523, 1359,1265,1180,1167,1141,907,877,816,810,754,684,672,535,509cm-1. EI-MS m/z 237(1), 235(2,[M]+), 136(100), 90(48), 64(23). EI-HRMS m/z calculated value [M] + C7H6ClNO4S 234.9706, measured value 234.9707.
Main reference materials
[1]Journal of the American Chemical Society,Volume133,Issue40,Pages15906-15909



 微信扫一扫打赏
微信扫一扫打赏
