Background and overview[1][2]
2,3-Dihydro-2-methylbenzofuran is an organic intermediate that can be prepared from 2-allylphenol.
Preparation method[1-2]
1. Cyrine Ayed et al. reported that 2,3-dihydro-2-methylbenzofuran can be synthesized from the following raw material (E)-2-(prop-1-en-1-yl)phenol.
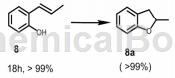
General procedure: Add 0.1 mmol of styrene or its derivatives or olefins, photocatalyst (10 mg) to a glass vial equipped with an oxygen balloon containing 1.5 mL of solvent. A blue LED lamp (460nm, 0.065Wcm-2, OSA Opto Light GmbH) was used as the light source. The reaction mixture was stirred at room temperature
Temperature and irradiation for 18 hours. Conversion and selectivity determined by GC-MS.
2. Zhu X et al. reported the preparation of 2,3-dihydro-2-methylbenzofuran using 2-allylphenol under the action of catalyst II.
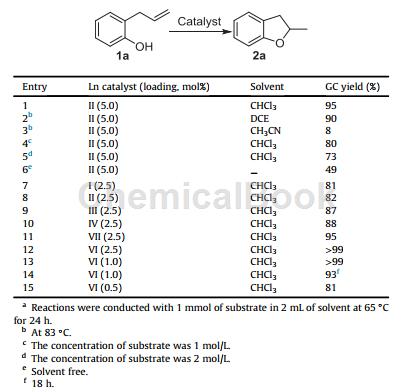
To a 10 mL Schlenk tube, add II (0.0265 g, 0.025 mmol), DCE (2.0 mL) and 2-allylphenol (0.10 mL, 1.0 mmol) under dry argon. The resulting mixture was stirred at 83°C for 24 hours. The reaction was cooled to room temperature and the catalyst was removed by filtration through a short pad of silica gel. Product 2a was obtained by silica gel column chromatography using pentane/diethyl ether or petroleum ether/ethyl acetate as the eluent. The yield determined by GC using nonane as internal standard was 90%. 1H NMR (400 MHz, CDCl3): d ¼ 7.27e7.20 (m, 2 H), 6.97e6.89 (m,2 H), 5.05 e4.96 (m, 1 H), 3.41e3.35 (dd, J ¼ 15.6, 8.8 Hz, 1 H), 2.93e2.87 (dd, J ¼ 15.2, 7.6 Hz, 1 H), 1.58 (d, J ¼ 6.4 Hz, 3 H). 13C NMR (100 MHz, CDCl3): d ¼ 154.5, 122.9, 121.9, 119.9, 115.1, 104.2, 74.3, 32.0, 16.7.
Main reference materials
[1] Cyrine A, Lucas C D S, Di W, et al. Designing conjugated microporous polymers for visible light-promoted photocatalytic carbon-carbon double bond cleavage in aqueous medium[J]. Journal of Materials Chemistry A, 2018:10.1039 .C8TA05772A-.
[2] Zhu



 微信扫一扫打赏
微信扫一扫打赏
