Background and overview[1][2]
Me-fluoroanisole is an important chemical intermediate, mainly used in the preparation of liquid crystal, pharmaceutical, and pesticide intermediates.
Preparation[1]
At present, there are two main processes for preparing m-fluoroanisole reported in the literature: one is to use m-aminoanisole through the Seaman reaction to prepare m-fluoroanisole, and the reaction equation is:
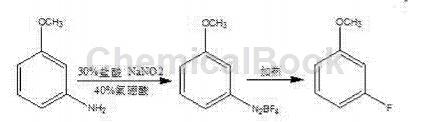
The synthesis method of this process has dangerous pyrolysis process, harsh operating conditions, difficult industrialization, low overall reaction yield, and high unit consumption.
CN201610592234.9 provides a preparation method of m-fluoroanisole with low production cost and easy industrialization. The present invention is realized through the following technical solutions:
A method for preparing m-fluoroanisole is special in that it includes the following steps:
(1) Add methanol to the reaction kettle, raise the temperature to 20~60°C, add potassium hydroxide, and keep warm;
(2) Continue to add dimethyl sulfoxide and cuprous chloride to the reaction kettle, raise the temperature, add m-difluorobenzene and react for 14 to 18 hours. The reaction equation is:
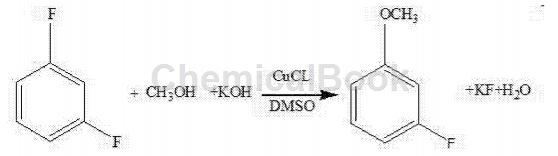
(3) Add water to the reaction product to distill it to obtain a crude product, which is further distilled to obtain m-fluoroanisole.
The beneficial effects of the present invention are: the present invention uses m-difluorobenzene as raw material to prepare m-fluoroanisole by a one-step etherification method. The raw materials are easy to obtain and cheap, have few steps, are simple to operate, do not require high-pressure equipment, and greatly reduce the cost. The raw material cost and production cost are reduced, and the product yield can reach more than 80%, which is much higher than the yield obtained by previous processes, and is suitable for large-scale industrial production.
Apply[2-3]
Pesticides refer to a class of drugs used in agricultural production to kill insects, bacteria, and harmful animals (or weeds) in order to ensure and promote the growth of plants and crops. Specifically refers to pesticides used in agriculture to prevent and control diseases and pests, regulate plant growth, and eliminate weeds. Existing pesticides tend to accumulate in plants and the human body, posing potential safety hazards. Therefore, it is necessary to provide an environmentally friendly and pollution-free pesticide formula to meet the needs of use.
CN201710314064.2 provides an environmentally friendly and pollution-free pesticide formula, which consists of the following raw materials in parts by weight: 10 to 18 parts of dinotefuran, 10 to 15 parts of pyraclostrobin, and 20 to 27 parts of fludioxonil , 10 to 15 parts of fomesafen, 10 to 20 parts of bifenthrin, 5 to 12 parts of castor oil, 2 to 10 parts of m-fluoroanisole, 6 to 14 parts of propyl spinosad, and sagebrush 10~17 parts of skin, 10~15 parts of diclosulam, 3~8 parts of 2-fluoronaphthalene and 1~5 parts of stabilizer. This kind of environmentally friendly and pollution-free pesticide has a reasonable proportion of ingredients, is safe and non-toxic, easy to store, does not pollute the environment, has good insecticidal effect, long shelf life and low production cost.
Arteriosclerosis is the main cause of many diseases and deaths, especially in developed countries. Increasing high-density lipoprotein can effectively reduce sudden cerebrovascular diseases. One of the most effective drugs currently is Anacetrapib, which is used clinically as a cholesteryl ester transfer protein (CETP) inhibitor. 4-Fluoro-5-isopropyl-2-methoxyphenylboronic acid is an important structural unit of acetrapib. CN201610011058.5 discloses a method for synthesizing 4-fluoro-5-isopropyl-2-methoxyphenylboronic acid. Starting from m-fluoroanisole, it reacts with iodine to undergo iodination, followed by catalytic coupling with isopropylboronic acid, and finally selective deprotonation and boronization under TEMPLi or TEMPMgCl conditions, and the product is obtained after hydrolysis.
The first step: add m-fluoroanisole, hydrogen peroxide and ethanol and stir evenly, then add iodine in batches. After the dropwise addition, the reaction solution is dissolved. Continue to keep the reaction for 1-5 hours until the detection reaction is complete. Add iodine. NaHSO3 quenched the reaction, layered with ethyl acetate, washed the organic layer with saturated sodium chloride, and distilled to dryness to obtain 2-fluoro-4-methoxyiodobenzene;
Second step: After adding 2-fluoro-4-methoxyiodobenzene, isopropylboronic acid, alkali aqueous solution and DME to the reaction bottle, nitrogen is blown under the page to remove oxygen, and finally the catalyst is added, and the temperature is raised to 80°C to 100°C to react. After the reaction, separate layers. The aqueous layer is extracted again with ethyl acetate. The organic layers are combined and washed with saturated sodium chloride. After the solvent is evaporated to dryness, toluene is added and distilled again to obtain 2-fluoro-4-methoxy. cumene;
Step 3: Add anhydrous solvent and 2,2,6,6-tetramethylpiperidine, control the temperature to -78oC to -20oC, add n-butyllithium or isopropylmagnesium chloride dropwise, and then add 2 -Fluoro-4-methoxycumene. After deprotonation is completed, add trimethyl borate. After the reaction is completed, add hydrochloric acid to quench, separate the layers, extract with ethyl acetate three times, combine the organic layers, evaporate to dryness, add methanol to dissolve, then add water to precipitate, methyl tert. Butyl ether and n-heptane were mixed with a solvent to obtain an off-white solid product, 4-fluoro-5-isopropyl-2-methoxyphenylboronic acid, with a three-step yield of 42-48%.
The synthesis route of this method is reasonably designed. m-fluoroanisole is used as the starting material to complete the synthesis of 4-fluoro-5-isopropyl-2-methoxyphenylboronic acid in a three-step method. In the first step, large The sterically hindered iodine improves the selectivity during halogenation. In the third step, a large sterically hindered base is used to deprotonate to improve the deprotonation selectivity, thus ensuring the efficiency of the entire synthesis.
Main reference materials
[1] CN201610592234.9 Preparation method of m-fluoroanisole
[2] CN201710314064.2 An environmentally friendly and pollution-free pesticide formula
[3] CN201610011058.5 A method for synthesizing 4-fluoro-5-isopropyl-2-methoxyphenylboronic acid



 微信扫一扫打赏
微信扫一扫打赏
