Background and overview[1]
(4,8-bis(4-chloro-5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-B4,5-B’]dithiophene-2,6- Diyl) bistrimethyltin can be used as a pharmaceutical synthesis intermediate. If you inhale (4,8-bis(4-chloro-5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-B4,5-B’]dithiophene-2,6-bis (Bit) bistrimethyltin, please move the patient to fresh air; if the skin comes into contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; if the eyes come into contact, seek medical attention. Separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1]
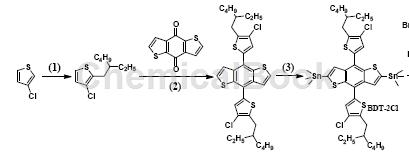
(4,8-bis(4-chloro-5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-B4,5-B’]dithiophene-2,6- The preparation of diyl)bistrimethyltin is as follows: Step 1: 1) Use 3-chloro-thiophene as raw material, THF as solvent, add LDA, react at 0°C for 2 hours; then add 2-ethylhexyl bromide, 60°C, overnight. (2) Dissolve 3-chloro-2-(2-ethylhexyl)-thiophene prepared in step 1 in THF, add n-BuLi, and react at 0°C for 2 hours; add benzo[1,2-b:4 , 5-b’]bithiophene-4,8-dione, react at 50°C for 2 hours; add SnCl2·2H2O, HCl, room temperature, and then 50°C overnight. (3) Add LDA and THF to the intermediate prepared in step 2 and react at -78°C for 4 hours; add Sn(CH3)3Cl and heat from -78°C to room temperature overnight to prepare (4,8-di(4) -Chloro-5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-B4,5-B’]dithiophene-2,6-diyl)bistrimethyltin
Apply[1]
(4,8-bis(4-chloro-5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-B4,5-B’]dithiophene-2,6- Diyl)bistrimethyltin can be used to synthesize the following compounds:
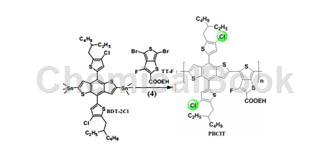
To (4,8-bis(4-chloro-5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-B4,5-B’]bithiophene-2,6 -Diyl)bistrimethyltin is prepared by adding TT-F, adding Pd(PPh3)4, dissolving in anhydrous toluene/DMF, and reacting at 120°C.
Main reference materials
[1] rination of Side Chains: A Strategy for Achieving a High Open Circuit Voltage Over 1.0 V in Benzo[1,2-b:4,5-b#]dithiophene-Based Non-Fullerene Solar Cells



 微信扫一扫打赏
微信扫一扫打赏
