Background
2,6-Difluoroacetophenone is an intermediate in organic synthesis and can be used in laboratory research and development processes and chemical and pharmaceutical synthesis processes.
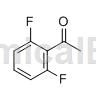
Preparation [1][2]
1.2. Preparation of 6-difluoroacetophenone: hydrolysis with sulfuric acid/acetic acid
Magnesium chloride (167g, 1.75mol) was added to a solution of diethyl malonate (125g, 780mmol) in chlorobenzene (500mL) and the slurry was stirred at ambient temperature for 30 minutes. With the outside cooling, keeping the internal temperature between 25-27°C during the addition, triethylamine (238 mL, 1.71 mol) was added. Stir the slurry for 30 minutes at ambient temperature. With external cooling, maintaining the temperature between 25-27°C during the addition, a solution of 2,6-difluorobenzoyl chloride (100 g, 565 mmol) in chlorobenzene (100 mL) was slowly added. The slurry was stirred at ambient temperature for 2 hours and then cooled to 0°C. The slurry was poured into IN hydrochloric acid (2000 mL). The two-phase mixture is allowed to return to ambient temperature and the phases are allowed to separate. The phases are separated. To a portion of the chlorobenzene phase (76 g) was added a mixture of concentrated sulfuric acid (10 mL) and 60% aqueous acetic acid (35 mL). The mixture was heated to 91-94°C for 7 hours, cooled to ambient temperature, and then adjusted to pH 7 with 10% aqueous sodium hydroxide. The phases were separated and the aqueous phase was back-extracted with chlorobenzene. The chlorobenzene phases were combined and washed with water. HPLC weight % analysis of the combined chlorobenzene phases showed a yield of 7.57 g (87%) of 2,6-difluoroacetophenone.
2.2. Preparation of 6-difluoroacetophenone: hydrolysis with acetonitrile/water
Magnesium chloride (1.65 g, 17.3 mmol) was added to a solution of diethyl malonate (1.24 g, 7.7 mmol) in ethyl acetate (20 mL) and the slurry was stirred at ambient temperature for 30 minutes. Triethylamine (2.35 mL, 16.7 mmol) was added and the slurry was stirred for another 30 minutes. The slurry was cooled to 0°C and a solution of 2,6-difluorobenzoyl chloride (1.0 g, 5.6 mmol) in ethyl acetate (5 mL) was added dropwise over 15 minutes, keeping the internal temperature below 5°C. At the end of the addition, the reaction was allowed to warm to ambient temperature and stirred for approximately 3 hours. The slurry was then treated with IN hydrochloric acid (50 mL) and extracted with ethyl acetate (100 mL). The organic phase was separated, dried over MgSO4, and filtered. The filtrate was concentrated under reduced pressure to yield a colorless oil (1.97 g) containing the intermediate. The oil was dissolved in acetonitrile (25 mL) and water (2 mL) was added. The solution was transferred to the pressure reactor and sealed. The intermediate solution was stirred and heated to 150°C for 1 hour. The reaction mixture was cooled to ambient temperature and residual pressure released. HPLC weight % analysis of the solution showed a yield of 874 mg of 2,6-5 fluoroacetophenone (100%).
3. Preparation of 2,6-difluoroacetophenone using monoethyl malonate potassium salt
Combine potassium monoethyl malonate (13.4g, 77mmol), magnesium chloride (16.5g, 173mmol), ethyl acetate (40mL) and tetrahydrofuran (60mL) and stir at ambient temperature for 30 minutes. The reaction mixture was cooled to 0°C and triethylamine (23.5 mL, 167 mmol) was added. The reaction slurry was heated to 50°C and held for 1 hour, then cooled back to 0°C. Over 55 min, a solution of 2,6-difluorobenzoyl chloride (10.0 g, 56 mmol) in ethyl acetate (25 mL) was slowly added to the slurry, maintaining the internal temperature below 2°C. At the end of the addition, the reaction was allowed to warm to ambient temperature and stirred for 19 hours. The reaction was cooled to 0°C and treated with IN hydrochloric acid (200 mL). The clear biphasic mixture was returned to ambient temperature and additional ethyl acetate (100 mL) was added. The phases were separated and the organic phase was dried over MgSO4, filtered and the filtrate was concentrated under reduced pressure to yield a yellow oil residue containing the intermediate (15.46 g). The oil was dissolved in acetonitrile (100 mL) and water (5 mL) and transferred to a pressure reactor with condenser and back pressure regulator. The reaction mixture was sealed in a pressure reactor, stirred and heated to 150°C for 1 hour. The reaction was cooled to ambient temperature and residual pressure released. HPLC weight % analysis of the reaction solution showed a yield of 8.60 g (99%) of 2,6-difluoroacetophenone.
4. Preparation of 2,6-difluoroacetophenone using pyridine as base
Magnesium chloride (1.65 g, 17.3 mmol) was added to a solution of diethyl malonate (1.24 g, 7.7 mmol) in chlorobenzene (20 mL) and the slurry was stirred at ambient temperature for 30 minutes. Pyridine (1.35 mL, 16.7 mmol) was added and the slurry was stirred for another 30 minutes. The reaction was cooled to 0°C, and 2,6-dioic acid was added dropwise over approximately 10 min.� A solution of benzoyl chloride (1.0 g, 5.6 mmol) in chlorobenzene (5 mL), keeping the internal temperature below 1°C. The reaction was allowed to warm to ambient temperature and stir for approximately 21 hours. The reaction mixture was treated with IN hydrochloric acid (20 mL) and diluted with water (80 mL). The phases were separated and the chlorobenzene (bottom) phase was transferred to the pressure reactor. Water (2 mL) was added to the reactor and the reactor was sealed. The reaction mixture was stirred and heated to 150°C for 1 hour. The reaction was cooled to ambient temperature and residual pressure released. The reaction mixture was diluted with additional water and chlorobenzene and the phases were allowed to separate. The chlorobenzene (bottom) phase containing the title compound was isolated. HPLC weight % analysis of the chlorobenzene phase showed a yield of 505 mg of 2,6-difluoroacetophenone (58%).
5. Preparation of 2,6-difluoroacetophenone using 2,6-dimethylpyridine as a base
Magnesium chloride (1.65 g, 17.3 mmol) was added to a solution of diethyl malonate (1.24 g, 7.7 mmol) in chlorobenzene (20 mL) and the slurry was stirred at ambient temperature for 30 minutes. 2,6-lutidine (1.93 mL, 16.7 mmol) was added and the slurry was stirred for another 30 minutes. The reaction was cooled to 0°C and a solution of 2,6-difluorobenzoyl chloride (1.0 g, 5.6 mmol) in chlorobenzene (5 mL) was added dropwise over approximately 10 minutes, maintaining the internal temperature below 1°C. The reaction was allowed to warm to ambient temperature and stir for approximately 24 hours. The reaction was treated with IN hydrochloric acid (50 mL) and diluted with water (50 mL). The phases were separated and the chlorobenzene (bottom) phase was transferred to the pressure reactor. Water (2 mL) was added to the reactor and the reactor was sealed. The reaction mixture was stirred and heated to 150°C for 1 hour. The reaction was cooled to ambient temperature and residual pressure released. The reaction mixture was diluted with additional water and chlorobenzene and the phases were allowed to separate. The chlorobenzene (bottom) phase containing the title compound was isolated. HPLC weight % analysis of the chlorobenzene phase showed a yield of 859 mg of 2,6-difluoroacetophenone (99%).
6. Preparation of 2,6-difluoroacetophenone using 2-methylpyridine as a base
Magnesium chloride (1.65 g, 17.3 mmol) was added to a solution of diethyl malonate (1.24 g, 7.7 mmol) in chlorobenzene (20 mL) and the slurry was stirred at ambient temperature for 30 minutes. 2-methylpyridine (1.68 mL, 16.7 mmol) was added and the slurry was stirred for another 30 minutes. The reaction was cooled to 0°C and a solution of 2,6-difluorobenzoyl chloride (1.0 g, 5.6 mmol) in chlorobenzene (5 mL) was added dropwise to the reaction over approximately 10 min, maintaining the internal temperature below 1°C. The reaction was allowed to warm to ambient temperature and stir for approximately 24 hours. The reaction was treated with IN hydrochloric acid (50 mL) and diluted with water (50 mL). The phases were separated and the chlorobenzene (bottom) phase was transferred to the pressure reactor. Water (2 mL) was added to the reactor and the reactor was sealed. The reaction mixture was stirred and heated to 150°C for 1 hour. The reaction was cooled to ambient temperature and residual pressure released. The reaction mixture was diluted with additional water and chlorobenzene and the phases were allowed to separate. The chlorobenzene (bottom) layer containing the title compound was separated. HPLC weight % analysis of the chlorobenzene phase showed a yield of 697 mg of 2,6-difluoroacetophenone (80%).
7. Preparation of 2,6-difluoroacetophenone using N,N-diethylaniline as base
Magnesium chloride (1.65 g, 17.3 mmol) was added to a solution of diethyl malonate (1.24 g, 7.7 mmol) in chlorobenzene (20 mL) and the slurry was stirred at ambient temperature for 30 minutes. N,N-diethylaniline (2.65 mL, 16.7 mmol) was added and the slurry was stirred for another 30 minutes. The reaction was cooled to 0°C and a solution of 2,6-difluorobenzoyl chloride (1.0 g, 5.6 mmol) in chlorobenzene (5 mL) was added dropwise over 10 minutes, keeping the internal temperature below 1°C. The reaction mixture was allowed to warm to ambient temperature and stirred for 22 hours. The reaction was treated with IN hydrochloric acid (50 mL) and diluted with water (50 mL). The phases were separated and the chlorobenzene (bottom) phase was transferred to the pressure reactor. Water (2 mL) was added to the reactor and the reactor was sealed. The reaction mixture was stirred and heated to 150°C for 1 hour. The reaction was cooled to ambient temperature and residual pressure released. The reaction mixture was diluted with additional water and chlorobenzene and the phases were allowed to separate. The chlorobenzene (bottom) phase containing the title compound was isolated. HPLC weight % analysis of the chlorobenzene phase showed a yield of 876 mg of 2,6-difluoroacetophenone (100%).
8. Preparation of 2,6-difluoroacetophenone using tributylamine as base
Magnesium chloride (1.65 g, 17.3 mmol) was added to a solution of diethyl malonate (1.24 g, 7.7 mmol) in chlorobenzene (20 mL) and the slurry was stirred at ambient temperature for 30 minutes. Tributylamine (1.98 mL, 16.7 mmol) was added and the slurry was stirred for another 30 minutes. The reaction was cooled to 0°C and a solution of 2,6-difluorobenzoyl chloride (1.0 g, 5.6 mmol) in chlorobenzene (5 mL) was added dropwise to the reaction over approximately 10 minutes, maintaining the internal temperature below 1°C. The reaction was allowed to warm to ambient temperature and stirred for 22 hours. The reaction mixture was treated with IN hydrochloric acid (50 mL) and diluted with water (50 mL). The phases were separated and the chlorobenzene (bottom) phase was transferred to the pressure reactor. Water (2 mL) was added to the reactor and the reactor was sealed. The reaction was stirred and heated to 150°C for 1 hour. The reaction was cooled to ambient temperature and residual pressure released. The reaction mixture was diluted with additional water and chlorobenzene and the phases separated. The chlorobenzene (bottom) phase containing the title compound was isolated. HPLC weight % analysis of the chlorobenzene phase showed a yield of 701 mg of 2,6-difluoroacetophenone (81%).
Main reference materials
[1] Chang Yu, Wang Mingwen, & Wang Xiulan. (2001). Synthesis of 2-chloro-2′,4′-difluoroacetophenone. Chinese Journal of Medicinal Chemistry, 11(5), 287-290 .
[2] Wang Xiulan, & Chang Yu. (2002). Aluminum trichloride catalytic compound 2-chloro-2′,4′-difluoroacetophenone. Journal of Taiyuan University of Technology, 33(3), 309- 311.
Compound 2—Chloro-2′,4′-difluoroacetophenone. Journal of Taiyuan University of Technology, 33(3), 309-311.



 微信扫一扫打赏
微信扫一扫打赏
