Background and overview[1]
2,3-Diamino-6-fluorotoluene can be used as a pharmaceutical synthesis intermediate. If 2,3-diamino-6-fluorotoluene is inhaled, please move the patient to fresh air; if there is skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; If eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1]
The preparation of 2,3-diamino-6-fluorotoluene is as follows:
Step 1: Synthesis of N-(3-fluoro-2-methylphenyl)acetamide
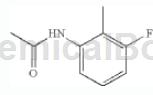
Dissolve 1-amino-3-fluoro-2-methylbenzene (1 equiv) in dichloromethane and slowly add acetic anhydride (2.0 equiv). The solution was stirred at room temperature for 4 hours. The reaction mixture was then quenched with water and the aqueous layer was extracted with ethyl acetate. Wash the organic layer with H2O, 10% HCl solution, H2O and brine. It was then dried over Na2SO4 and concentrated in vacuo to give N-(3-fluoro-2-methylphenyl)acetamide as a pink solid. LC/MS (m/z) 168.2 (MH+), Rt 1.91 minutes.
Step 2: Synthesis of 1-amino-3-fluoro-2-methyl-4-nitrobenzene
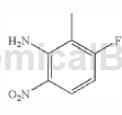
Add the mixture of HNO3/H2SO4 (1:1, 60% HNO3: concentrated H2SO4) cooled to 0°C dropwise into N-(3-fluoro-2-methylphenyl)acetamide to form 0.16M solution. The solution was stirred at 0°C for 10 minutes and then at room temperature for 30 minutes. The solution was then diluted with water and made alkaline (pH=10) by adding 6NNaOH. The mixture was then extracted with CH2Cl2 (3x), dried over Na2SO4, and concentrated in vacuo to give 1-amino-3-fluoro-2-methyl-6-nitrobenzene (the acetyl group was removed during basic workup). ). LC/MS (m/z) 171.1 (MH+), Rt 1.87 minutes.
Step 3: Synthesis of 2,3-diamino-6-fluorotoluene
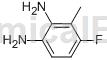
Suspend 1-amino-3-fluoro-2-methyl-6-nitrobenzene (1.0 equiv) and 10% Pd/C (0.1 equiv) in absolute ethanol at room temperature. The reaction flask was evacuated and then filled with H2. The resulting mixture was then stirred overnight under a hydrogen atmosphere. The resulting solution was filtered through celite and concentrated under vacuum to give 2,3-diamino-6-fluorotoluene. LC/MS (m/z) 141.1 (MH+), Rt 0.43 minutes.
Main reference materials
[1]US20060079564 Indazole benzimidazole compounds



 微信扫一扫打赏
微信扫一扫打赏
