Background and overview[1][2]
M-Toluidine is a colorless liquid that turns yellow or reddish brown in the air or under the influence of light. The relative density is 0.989, the melting point is -43.6℃, and the boiling point is 203~204℃. Slightly soluble in water, soluble in ethanol and ether. It can evaporate together with water vapor and is produced by the reduction reaction of m-nitrotoluene. Meta-toluidine is mainly used as a pharmaceutical synthesis intermediate.
Preparation[2]
Add a certain amount of industrial methanol solvent, catalyst and m-nitrotoluene in sequence to the high-pressure reaction kettle, and replace them with hydrogen three times. After the replacement is qualified, turn on the stirrer at a stirring speed of 1200~1500r/min, introduce hydrogen gas, slowly heat the reaction kettle to the required temperature, and pressurize. Monitor the progress of the hydrogenation reaction by observing and recording the changes in pressure within the reactor over time. After the reaction for a period of time, stop the flow of hydrogen, cool, let it stand, let the catalyst sink to the bottom of the kettle, absorb the supernatant (i.e., crude product), and analyze it through a gas chromatograph. The oxygen in the mixed gas can be absorbed by the alkaline pyrogallic acid solution. According to the change in the volume of the mixed gas before and after absorption, the oxygen content in the gas can be calculated. After adding an appropriate amount of air to flammable hydrogen, explode it, and calculate the hydrogen content in the gas based on the decrease in volume of the product after the explosion.
Oxygen absorption:
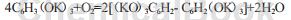
Hydrogen explosion:
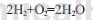
Use nickel as a catalyst to catalyze the hydrogenation reaction of m-nitrotoluene in industrial methanol solvent to generate m-toluidine. Through single factor examination and orthogonal experiments, using 80g of m-nitrotoluene as the calculation benchmark and under the fixed conditions of 200mL of industrial methanol solvent, the optimal reaction conditions were: 2.0g of catalyst, 6h of reaction time, and 6h of reaction temperature. 90℃, reaction pressure is 1.6MPa. The m-toluidine content obtained under these conditions is 99.79%, the light component content is 0.11%, and the heavy component content is 0.10%.
Apply[3-9]
M-Toluidine is mainly used as a pharmaceutical synthesis intermediate. Examples of its application are as follows:
1) Production of m-cresol. Add a certain concentration of sulfuric acid or hydrochloric acid and m-toluidine into the batching pot in proportion to form m-toluidine sulfate or m-toluidine hydrochloride. The m-toluidine sulfate or m-toluidine hydrochloride comes out of the batching pot. The aqueous solution is sent to the autoclave, the reaction temperature is controlled, m-toluidine sulfate or m-toluidine hydrochloride is hydrolyzed, and part of the water in the hydrolyzate is recovered through flash evaporation for ingredient use. The ammonia aqueous solution or caustic alkali aqueous solution neutralizes the hydrolyzate to a pH of After 3-5, it is extracted by organic solvent, and then the extraction agent is recovered and the crude m-cresol is refined. The method of one-step hydrolysis greatly reduces the amount of sulfuric acid and does not require the use of sodium nitrite. The concentration of dilute sulfuric acid produced during the reaction is less than 2%. The neutralization cost of dilute acid is low and the environmental pollution is small.
2) Prepare N, N’-bis(m-tolyl)urea. The method steps are as follows:
1) After mixing m-toluidine and water, add inorganic acid dropwise for a salt-forming reaction. After the dripping is completed, add urea and stir; the equivalent ratio of m-toluidine to inorganic acid is 1:0.95~1.05. Moles of urea�� is 1:0.5~1, and the salt-forming reaction temperature is 0~80℃;
2) Add the above reaction solution into the high-pressure reaction kettle, raise the pressure of the high-pressure reaction kettle to 2~10atm, keep it for 1-5 hours, then release the pressure, discharge, centrifuge, and wash with water. The invention has the advantages of low and easy-to-obtain raw materials, simple reaction, high overall yield and low production cost, and is suitable for industrial production. Based on m-toluidine, the molar yield of N, N'-di(m-toluidine) urea can reach more than 90% of the theoretical amount; after the reaction, the molar yield of the mother liquor can reach more than 95%.
3) Prepare m-fluorotoluene. This method has fewer steps, high yield, and high purity of the product obtained. The preparation method of m-fluorotoluene of the present invention includes the following steps:
1) Salt formation step: Mix anhydrous hydrofluoric acid and m-toluidine with a molar ratio of (2-5):1. The mixing method is to add dropwise to the anhydrous hydrofluoric acid at a constant rate within 7-10 hours. After the dropwise addition of all m-toluidine, react at 5-7°C for 1-3 hours to obtain mixture 1;
2) Diazotization step: Add sodium nitrite to mixture 1. The molar ratio of sodium nitrite to m-toluidine used in the salt-forming step is (1-1.5):1. Add sodium nitrite. In order to drop all the sodium nitrite into the mixture 1 at a uniform speed within 8-10 hours, after adding the sodium nitrite, react at 0-3°C for 1-3 hours to obtain the mixture 2;
3) Thermal decomposition step: Heat and decompose mixture 2 at 0-50°C.
4) The process of preparing m-cresol from a nitrogen oxide gas is to pass the nitrogen oxide gas into m-toluidine sulfate for diazotization, and then hydrolyze it in the presence of organic solvents and acidic conditions. The acid phase After flash evaporation under negative pressure, it is reused in the salt-forming process. The organic phase is delighted and distilled to obtain the m-cresol product. The present invention uses nitrogen oxide gas as the diazotization reagent, and there is no inorganic salt in the waste acid, making it possible to apply the waste acid in industry; the hydrolysis is carried out in the presence of an organic solvent, so that the m-cresol produced by the hydrolysis can be quickly dissolved into In the organic phase, the by-product tar-like substance produced by the reaction is very small, which improves the yield of m-cresol; the salt-free waste acid is flashed under negative pressure at a certain temperature to remove the reaction water and also strip out the organic matter, which not only makes the sulfuric acid Applying the same method and reducing the amount of sulfuric acid, the m-cresol product prepared by the process of the present invention has a purity of 99.7% and a yield of more than 90%.
Preparing a solvent blue 63 dye, which includes the following steps:
(a) Add m-toluidine to the reaction vessel, add 1-methylamino-4-bromoanthraquinone, alkali metal hydroxide and copper salt in sequence under stirring conditions, and then raise the temperature to 100~130°C to react 4-7 hours to the end point; the mass ratio of the 1-methylamino-4-bromoanthraquinone, m-toluidine, alkali metal hydroxide and copper salt is 6:12~16:3~4:0.04~0.08;
(b) Cool the product of step (a) to 60~70℃, then add methanol for isolation; after stirring, cool to 30~50℃, filter, wash and dry. The obtained reaction has high purity, few side reactions, and short reaction time; more importantly, the product has obvious crystallization, is easy to wash, and has less production and washing waste water.
5) Prepare 3-methylphenylhydrazine hydrochloride, including the following steps:
(1) Using m-toluidine as the starting material, diazobenzene chloride is prepared through diazotization reaction;
(2) The diazobenzene chloride obtained in (1) is further reduced under the action of a reducing agent to obtain a reduction product;
(3) The reduction product obtained in step (2) undergoes a hydrolysis reaction under the action of hydrochloric acid to generate the 3-methylphenylhydrazine hydrochloride. In particular, in step (2), the reduction reaction uses sodium metabisulfite. As a reducing agent, it is carried out under the conditions of 15~25℃ and pH 7~9. This method can shorten the production cycle and reduce production costs while having a higher yield.
6) Preparation of 3-methyl-4-isopropylphenol, the method is:
(1) Dissolve m-toluidine in sulfuric acid and react with isopropylation reagent to obtain 3-methyl-4-isopropylaniline. The isopropylation reagent is isopropyl alcohol, propylene or 2 -Chloropropane;
(2) The 3-methyl-4-isopropylaniline is then hydrolyzed to obtain a reaction solution containing 3-methyl-4-isopropylphenol. The hydrolysis is diazo hydrolysis or High temperature and high pressure hydrolysis using sulfuric acid as solvent;
(3) The reaction liquid containing 3-methyl-4-isopropylphenol obtained in step (2) is distilled and solvent refined to obtain needle-shaped crystalline 3-methyl-4-isopropylphenol; the present invention The method has the advantages of mild reaction conditions, high yield, few by-products, and little pollution. It effectively solves the problems of many by-products, harsh conditions, and large pollution in the existing preparation process of 3-methyl-4-isopropylphenol. problem.
Main reference materials
[1] Dictionary of Chemical Substances
[2] Research on the synthesis of m-toluidine by liquid phase hydrogenation of m-nitrotoluene
[3] CN200810235100.7 Method for producing m-cresol by direct hydrolysis
[4] CN200610051603.XN, aqueous high-pressure preparation method of N’-bis(m-tolyl)urea
[5] Preparation method of CN201210308373.6 m-fluorotoluene
[6] CN201019026062.9 A process for preparing m-cresol from nitrogen oxide gas
[7] CN201611074318.X Preparation method of solvent blue 63 dye
[8] CN200810021716.4 A preparation method of 3-methylphenylhydrazine hydrochloride
[9]CN201210534804.0 A kind of preparation method of 3-methyl-4-isopropylphenol



 微信扫一扫打赏
微信扫一扫打赏
