Background and overview[1-2]
P-Phenylphenol is an almost white crystal, insoluble in water, but soluble in ethanol, caustic soda solution and various organic solvents. It is obtained by the interaction of chlorobenzene and caustic soda solution under a certain temperature and pressure, and is refined by crystallization. Used as dye intermediates, resins, chemical reagents, fungicides, etc. Para-phenylphenol is an important new fine chemical intermediate and is widely used in dyes, pesticides, preservatives, coatings, printing and dyeing auxiliaries, photosensitive materials and other fields.
Industry insiders estimate that my country’s current potential market demand for phenylphenol is about 1,500 to 2,500t/a, so current demand mainly relies on imports. After joining the WTO, my country’s textile industry has gained rare development opportunities. my country’s textile industry will inevitably follow the path of scale and high-end development. In addition, my country has now become the world’s major producer of synthetic fibers. Therefore, including para-phenylphenol, The phenylphenol contained in it has good development space as a high-efficiency, high-grade, new printing and dyeing auxiliary. With the vigorous development of the information industry and the popularization of fax technology, the global market demand for heat- and pressure-sensitive dyes has grown rapidly, becoming a high value-added product with the greatest development potential in the dye industry. Industry insiders predict that my country’s annual demand for heat and pressure-sensitive dyes will grow at a double-digit rate in the next few years. At the same time, with the continuous development of its uses, the development prospects are extremely broad.
Apply[2]
1. Application in coating industry
P-Phenylphenol is mainly used to prepare p-phenylphenol formaldehyde resin. As a high-quality drying oil, it is used to prepare high-grade paints. This paint has extremely strong durability and weather resistance, and is especially suitable for humidity and cold. and offshore vessels.
2. Application in printing and dyeing industry
P-phenylphenol reacts with ethylene oxide to form polyoxyethylene ether. As a non-ionic emulsifier, polyoxyethylene ether can be used in pesticides and polyurethanes. It can also be used as a carrier (accelerator) for polyester dyeing and has good effects on fibers. Due to the puffing effect, at 95℃, the dyeing speed can be increased by 1,000 times. At the same time, p-phenylphenol can be used to make dye intermediates, such as red light sensitizing and green light sensitizing dyes in synthetic oxindol products. It is one of the main raw materials for color films and can be synthesized with organic silicon to make large-screen oil films for external light sources. Light valve, the big screen TV. Amide textiles are soaked in 130℃ water for 90 minutes. The water contains 4% dye 15510 and 7.5% p-phenylphenol (swelling agent). Wash with 150g/L ammonium sulfate and then85℃ for 2 minutes and dry. The dyed fabric did not fade in the fire performance test. Phenylphenol can also be used for dyeing artificial resins.
3. Application in heat-sensitive dyes
Thermal and pressure-sensitive copy paper has replaced the carbon oil-based copy paper that was widely used in the past abroad and domestically, and has been widely used in various aspects. Thermosensitive recording paper can be produced by mixing p-phenylphenol as a developer with certain fatty acids. The application of this developer improves the heat-matching properties of the recording paper. 10.3ml palmitic acid reacts with 1ml p-phenylphenol in the presence of PCl5 to obtain a certain resin. This resin is mixed with mica, CaCO3, polyvinyl alcohol silicone emulsion, fluorescein-containing dye, etc. The reaction mixture is coated on paper Go up and you will get the thermal recording paper.
This recording paper exhibits excellent heat fit properties. p-phenylphenol is mixed with bisphenol A as a color developer for thermal recording paper, and mixed with p-hydroxybenzoic acid resin in a ratio of (0.05~0.30):1. The mixture can be applied to the paper. Preparation of high-precision thermal recording paper. p-Phenylphenol is used as a color developer for pressure-sensitive copy paper to produce pressure-sensitive copy paper for confidential documents. The paper is coated with hot-melt ink. This ink contains 5% to 50% electron-receiving color developer wax. Melt 30 parts of p-phenylphenol in erucic acid amine. After melting, hot-melt ink is obtained. Apply ink on paper to form a solid ink layer, then place it on a flat piece of paper, and use a ballpoint pen to draw an image on the paper. This image is invisible to the naked eye. If the acetone solution of violet lactone is applied, a clear blue image will be obtained.
4. Synthesis of 8-hydroxyquinoline polymer
Under the catalysis of horseradish peroxidase, 8-hydroxyquinoline and p-phenylphenol undergo a copolymerization reaction. By adjusting the feeding ratio of the comonomer (8-hydroxyquinoline and p-phenylphenol) and the secondary content of The ratio of oxane to water can control the content of 8-hydroxyquinoline in the polymer. Thus, a method for synthesizing copolymers of different compositions was established.
Preparation[3]
P-phenylphenol production technology includes recovery of para-phenylphenol from the distillation residue of phenol produced by sulfonation method, diphenylsulfone alkali fusion method, biphenyl sulfonation method, cyclohexanone and phenol synthesis method, etc.
1. Recovery of p-phenylphenol when producing phenol by sulfonation method
During the production of phenol by alkali fusion through benzene sulfonation, mixed phenylphenol (a mixture of ortho- and para-phenylphenols) can be produced as a by-product due to the reaction between sodium phenolate molecules. Usually, the phenylphenol recovery process is to vacuum distill the distillation residue to separate the mixed phenylphenol fraction. The vacuum degree is 53.2~66.5Kpa and the temperature is 65~75℃. Start intercepting. Until 100℃ or above, but not exceeding 135℃, obtain phenol water containing no more than 10% phenol and then transfer to secondary distillation. Taking advantage of the difference in solubility of ortho- and para-phenylphenols in trichlorethylene, after cooling, para-phenylphenolThe phenol can be crystallized and separated from the solution, and then filtered and separated. The filter cake is qualified para-phenylphenol, and the ortho-phenylphenol remains in the mother liquor, so that the two can be separated. On the one hand, this method can effectively increase the efficiency of the device for producing phenol through alkali sulfonation of benzene, and on the other hand, it can reduce the pollution caused by the device to the environment. However, with the rapid development of my country’s petrochemical industry, the benzene sulfonation and alkali fusion production of phenol equipment, which currently accounts for 15% of the total production capacity, will gradually be replaced by the cumene method.
2. Diphenyl sulfone alkali fusion method
Japan’s Ube Kosan Company has applied for a patent on the alkali melting of diphenyl sulfone to produce ortho-phenylphenol and para-phenylphenol. The process is as follows: add potassium hydroxide heated to 340°C into the reactor, then add viscous diphenyl sulfone, and heat the reaction mixture at 340 to 370°C Em> Stir for 1 hour, then dissolve the cooled melt in water, acidify with hydrochloric acid to pH=5, extract and distill with ether to obtain a mixture of phenol, ortho-phenylphenol and para-phenylphenol. The total yield of reaction products was 91.2%.
3.Biphenyl sulfonation method
The production of para-phenylphenol by the biphenyl sulfonation alkali fusion method has been industrialized abroad for many years. For example, Germany’s Bayer Company, Japan’s Sanko Chemical Company, etc. have adopted this method for production. The technology is relatively mature, but this production method There are shortcomings such as too long route, harsh alkali fusion reaction conditions, large amounts of three wastes and low yield, which need to be further improved and perfected in production and practice. This method uses biphenyl as raw material, produces biphenyl-4-sulfonic acid through sulfonation, and then neutralizes it through alkali fusion to produce crude p-phenylphenol, and then sublimates it to obtain the product. The chemical reaction formula is as follows:
1) Sulfonation:
![]()
2) Alkali fusion:
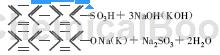
3) Neutralization:
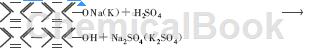
Main reference materials
[1] Dictionary of Chemical Substances
[2] Current status and application of p-phenylphenol technology
[3] New synthesis method of p-phenylphenol
Current status and application of technology
[3] New synthesis method of p-phenylphenol



 微信扫一扫打赏
微信扫一扫打赏
