Background and overview[1]
4-Diphenylmagnesium bromide can be used as a pharmaceutical synthesis intermediate. If 4-diphenylmagnesium bromide is inhaled, move the patient to fresh air; if the skin comes into contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable; if the eyes are clear, In case of contact, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1-2]
The preparation of 4-diphenylmagnesium bromide is as follows:
Method 1: Add 12.5g 4-bromobiphenyl, 27.38g magnesium, and 50g tetrahydrofuran into the reactor, add 0.125g elemental iodine particles for initiation, add 200g tetrahydrofuran, control the temperature at 40°C, and drop 237.5g 4-bromobiphenyl A mixed solution of benzene and 350g tetrahydrofuran was reacted for 2 hours and then cooled to 4°C to prepare 4-diphenylmagnesium bromide.
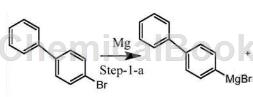
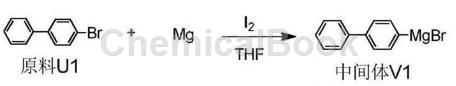
Method 2: In a 250mL three-necked flask, in a nitrogen atmosphere, add 0.05mol raw material U1 and 0.06mol Mg powder, dissolve them in 60ml dry tetrahydrofuran, add 0.0004mol elemental I2, heat to 40°C and stir until the solution turns from yellow It turns colorless. Heat the above mixed solution to 80°C and stir for 4 hours. No magnesium powder remains. The reaction is complete and the Grignard reagent 4-diphenylmagnesium bromide is generated. No purification is required, and the next step is carried out directly.
Apply[1-2]
4-Diphenylmagnesium bromide can be used as a pharmaceutical synthesis intermediate. If the following reaction occurs

Add 2g cuprous iodide to 4-diphenylmagnesium bromide, continue to cool to -15°C, dropwise add a mixture of 109g (S)-epichloropropane and 109g tetrahydrofuran, and stir for 2.0h to obtain the above compound.
In addition, 4-diphenylmagnesium bromide can also undergo the following reactions
The specific steps are: add 0.03 mol 9-fluorenone to a 250 mL three-necked flask in a nitrogen atmosphere, dissolve it with 40 ml dry tetrahydrofuran, slowly add the above format reagent intermediate V1 solution dropwise, and heat to reflux for 15 hours to generate A large amount of white precipitate was then cooled to room temperature, and saturated NHCl4 was added to convert the Grignard salt into alcohol; after the reaction was completed, it was extracted with ether, dried and evaporated, and passed through a silica gel column to obtain a slightly yellow solid tertiary alcohol intermediate W1, HPLC purity 99.2% , the yield is 72.5%.
Main reference materials
[1] CN201810029965.1 A synthesis method of LCZ696 intermediate
[2] CN201710383848.0 An organic compound with fluorene as the core and its application



 微信扫一扫打赏
微信扫一扫打赏
