Background and overview[1]
Accelerator m, as a super-fast accelerator for rubber, also has the function of plasticizer and is an important rubber additive. Accelerator m is widely used in the rubber industry with large consumption. It is also the matrix for the synthesis of other post-effect accelerators (such as sulfenamide accelerators). However, the current production process of accelerator M is deficient, especially the mother liquor wastewater and hydrogen sulfide gas generated during the production process are seriously polluted, and the post-processing link is weak, which seriously restricts its development. Industry experts believe that during the “Twelfth Five-Year Plan” period, my country should develop and promote clean production processes for additive M, strengthen the management of “three wastes” in the production process, and reduce raw material consumption. This is the fundamental way to promote the development of additive M production.
Properties[1]
The accelerator m has a thione structure. The reason is that in the infrared spectrum of this compound, there is no thiol absorption band at 2500~2600crn1. In 1993, the structural determination results of its derivatives showed that it was a sulfhydryl structure, which was in line with what people had already grasped in actual production. According to the structural theory of basic organic chemistry, there is often interconversion between the enol structure and the thione structure, and oxygen atoms can migrate within the molecule. Therefore, the accelerator m should actually be a resonance hybrid of the above two structures. Usually the infrared characteristic absorption band of the carbon-nitrogen double bond is located between 1640 and 1690cm. However, when the carbon-nitrogen double bond is conjugated with an adjacent group or forms a ring, due to the electron cloud transfer in the double bond, the stretching vibration frequency Red shift, peak shape broadens, its range is generally 1500 ~ 1670cm. In the accelerator m molecule, since the electronegativity of the sulfur atom is stronger than that of the carbon atom, and the sulfur atom itself has a lone pair of electrons, it will be formally conjugated with the carbon-nitrogen double bond, preventing the electrons from the carbon-nitrogen double bond to a certain extent. Due to the transfer caused by the conjugation of the benzene ring, the carbon-nitrogen double bond maintains a higher electron cloud density, which in turn causes the carbon-nitrogen double bond stretching vibration frequency in the accelerator m to be at a higher wave number, that is, l664cm. In the infrared spectrum of accelerator m, the characteristic absorption peak of thione group appears at 1050-1200 cm with high intensity, while the characteristic absorption peak of thiol group is not detected at 2500-2600 cm, which shows that accelerator m It does have a thione structure. However, under the action of a strong base, the accelerator m can form a sulfhydryl salt, indicating that it has a sulfhydryl structure. Therefore, the real structure of the accelerator m is a resonance hybrid of the enol structure and the thione structure. Accelerator m has good thiolation flatness and low vulcanization critical temperature (125℃). It can be widely used in various rubbers and has a wide vulcanization range. It can be used alone or in combination with thiocarbamates and thiuram. When used together with guanidine, guanidine and other alkaline accelerators, the dispersion effect is good. The accelerator M is less toxic, but has certain toxicity to human skin and respiratory system.
Apply[1]
1. Vulcanization accelerator
Accelerator m is a general-purpose rubber vulcanization accelerator. It is a semi-super accelerator that can be used alone or mixed with other accelerators. It has a rapid acceleration effect on natural rubber and general sulfur vulcanized synthetic rubber.
2. Accelerator matrix
Accelerator m is the parent material of most sulfenamide accelerators and is a widely used organic intermediate synthesis accelerator NOBS, Ds, Ns, cz, D1Bs, Dz, Mz (2-thiol benzo Thiazole zinc salt), DM, TBsliIV-tert-butyl~bis(2-benzothiazole)sulfenimide], cBBs[IV-cyclohexyl bis(2-benzothiazole)sulfenamide], etc., are all ionized Development of small liter accelerator m. The quality and output of accelerator m are directly related to the development of rubber.
3. Chemical plasticizer
In the process of rubber mastication, adding a small amount of accelerator m can improve the plasticity of rubber by forming a loose complex structure with sulfur, quickly reduce the Mooney viscosity of rubber, and speed up the mastication of raw rubber. speed, reduce the energy consumption of mastication, shorten the time of mastication, and greatly improve the efficiency of mastication.
4. Metal corrosion inhibitor
Accelerator M can also effectively prevent corrosion of metals, etc.” In various media, especially water, metal corrosion is often prevented by adding corrosion inhibitors, which is of great significance to the protection of cultural relics. It has been confirmed that benzazazole and some mercapto-substituted heterocyclic compounds, such as accelerator m, 2-mercaptobenzimidazole and 2-mercaptobenzoxazole, are effective corrosion inhibitors for metallic copper.
5. Mineral flotation agent
Another use of accelerator m is as a mineral flotation agent, mainly used as a capture agent for the flotation of sulfide ores such as silver, chalcopyrite, galena, pyrite and activated sphalerite, sometimes with disulfide Mixing substituted amino acid salts can further improve the yield and grade of flotation finished products.
6. Resin modifier
Accelerator m can also be used to modify epoxy resin. Adding a small amount of accelerator m to epoxy resin can significantly improve the bonding strength and curing rate of epoxy resin, and has the effect of significantly reducing the reaction temperature and shortening the curing reaction time. The larger the accelerator m, the faster the curing rate. This feature has important guiding significance for rapid industrial repair, especially online industrial repair.
7. Initiator
The accelerator m can act as an initiator, increasing the degree of polymerization of styrene and narrowing the relative molecular mass distribution of the polymer. The relative molecular mass of the polymer increases linearly with the increase in conversion rate, showing the behavior of benzene. Ethylene has “active” and controllable polymerization characteristics in the presence of accelerator m”2’4⋯. This is very important to the FRP industry, especiallyThe preparation of special materials in the aerospace industry has certain guiding significance.
8. Precious metal extraction agent
Accelerator m is used as an extraction agent and is currently mainly used for the separation of noble metals rhodium and iridium. The lone electrons on the sulfur and nitrogen atoms in the accelerator molecule are easily combined with the precious metals to solve the problem of difficulty in separating the two precious metals rhodium and iridium that has troubled people for a long time.
9. Others
Accelerator m can be used as a lubricant and adsorbent. It also has its special uses in electrochemistry. For example, adding a small amount of accelerator m to the electrode material can change the electrode performance, which is of great significance to the development of electrochemistry.
Preparation[2]
According to raw materials, there are more than 10 synthesis methods for accelerator m, such as aniline method (also called high pressure method), o-nitrochlorobenzene method (also called normal pressure method), N-methylaniline method, N , N-dimethylformamide method, benzothiazole method, etc., but most methods have not been industrialized due to low yield and high cost. The methods to achieve industrialization include aniline method and o-nitrochlorobenzene method. The o-nitrochlorobenzene method has been basically eliminated at home and abroad due to the high cost of raw materials and the large amount of wastewater with high salt content that is difficult to treat during the production process. At present, the production of accelerator m basically adopts the high-pressure synthesis method using aniline as raw material. There are two main high-pressure synthesis methods using aniline as raw material. One is the intermittent production method of pure aniline system (also called the stuffy pot method). Currently, most domestic manufacturers use this method; the other is to replace part of aniline with nitrobenzene. Continuous production method, according to reports, currently only Sinopec Nanjing Chemical Industry Co., Ltd. uses this method in China.
1. Aniline method (batch method)
The aniline method is to put a certain proportion of aniline, carbon disulfide, and sulfur into a high-pressure reactor at one time. After the temperature is raised, the reaction temperature is kept at 250-260°C and the pressure is 9-10MPa’F to react to generate crude accelerator m and waste gas. Hydrogen sulfide is then cooled down and the pressure is reduced. The exhaust gas hydrogen sulfide is purged and incinerated back to sulfur in a Claus incinerator. The crude accelerator m is purified by an acid-base method or a solvent method to obtain the finished accelerator m. This method has intermittent production, frequent operations, flammable and explosive problems, easy leakage of toxic and harmful gases, poor safety and poor environment; the material mixability is not good, and the yield based on aniline is low, about 85%. The reaction formula of aniline synthesis accelerator m is as follows:
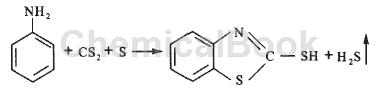
2. Aniline/nitrobenzene method (continuous method)
Aniline, nitrobenzene method (continuous method): aniline, nitrobenzene and recycled benzothiazole are mixed according to a certain mass ratio to prepare raw material NEX (the mass of nitrobenzene accounts for 0-40% of the mass of NEX) , carbon disulfide and sulfur are mixed to form the raw material CAS, and then the two materials are pumped into the high-pressure reactor in a certain proportion by a high-pressure pump, and react at a temperature of 220-280°C and a pressure of 7MPa to generate accelerator m, benzothiazole, and hydrogen sulfide The mixture is decompressed from the autoclave and discharged into the stripping tower. After stripping, the crude accelerator m is obtained from the liquid phase. The crude accelerator M is purified by an acid-base method or a solvent method to obtain the finished accelerator m; the gas phase After cooling and condensation, the thiazole and carbon disulfide are recovered, and the hydrogen sulfide gas is sent to the incineration equipment to prepare sulfuric acid and by-product steam. This method realizes continuous operation and safety interlocking, has a high degree of self-control, good production operating environment and safety, the mixing of materials is better than intermittent production, and realizes the recovery and application of benzothiazole. The yield based on aniline is At about 89%, we are currently exploring ways to enhance material mixing by adding a reactor stirring device, adjusting the proportion of nitrobenzene mass to NEx mass, and using catalysts that can lower temperature and pressure, increase yields, and reduce by-products to optimize production. process to further improve product yield. The reaction formula of aniline and nitrobenzene synthesis accelerator m is as follows:
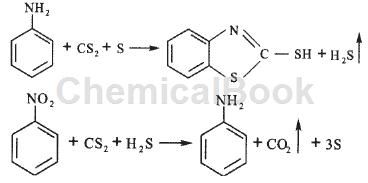
Main reference materials
[1] Synthesis and application progress of accelerator m
[2] Production technology of accelerator m



 微信扫一扫打赏
微信扫一扫打赏
