Background and overview[1]
4-n-Heptylbromobenzene can be used as a pharmaceutical synthesis intermediate. If 4-n-heptylbromobenzene is inhaled, please move the patient to fresh air; if the skin comes into contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel uncomfortable; if the eye contact , you should separate your eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse your mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1]
4-n-Heptylbromobenzene is prepared as follows:

The specific steps are as follows: Dissolve phenylheptane (100g, 0.57mol) in 900mL chloroform, and add ferric chloride (1.41g, 9mmol). The mixture was cooled to 0 °C and bromine (32 mL, 0.62 mmol) was added dropwise through a separate funnel. The reaction was kept in the dark to prevent bromination of aliphatic side chains. After stirring at room temperature overnight, the reaction was quenched with 1NKOH. After extraction, the organic phase was washed with water and dried over MgSO4. The crude product was a brown oily substance. It was purified by silica gel column chromatography using heptane as the eluent to obtain 60g of pure product 4-n-heptylbromobenzene as a light yellow oily substance at 42°C. %let. 1HNMR (CDCl3) δ (ppm): 0.88 (t, J=6.9Hz, 3H), 1.26-1.31 (m, 8H), 1.54-1.59 (m, 2H), 2.54 (t, J=7.8Hz, 2H) , 7.04 (d, J=8.2Hz, 2H), 7.38 (d, J=8.2Hz, 2H). FD-MS: m/z255 (M+).
Apply[1]
4-n-Heptylbromobenzene can be used as a pharmaceutical synthesis intermediate. If the following reaction occurs:
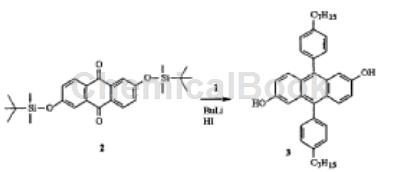
Step 1: Add 2,6-dihydroxyanthraquinone (80.0g, 0.33mol), imidazole (108.8g, 1.6mol), tert-butyldimethylsilyl chloride (115.5 g, 0.77mol) and DMF. 600ml. The dark red mixture was heated to 90°C for 3 hours. TLC indicated the reaction was complete. The reaction was cooled and poured into 2L cold water. The dark green needle-like precipitate was filtered off and washed with water and methanol. Dissolve the dark green crystals in ether and filter out the black insoluble portion. The bright yellow filtrate was concentrated and the crude product was suspended in boiling methanol. The yellow precipitate was filtered to obtain 85.1g of pure product in the form of yellow filamentous crystals with a yield of 54%. 1HNMR (CDCl3) δ (ppm): 0.28 (s, 12H), 1.00 (s, 18H), 7.14 (dd, J1=8.5Hz, J2=2.5Hz, 2H), 7.64 (d, J=2.5Hz, 2H ), 8.17 (d, J=8.5Hz, 2H). 13CNMR (CDCl3): -4.36, 25.53, 117.35, 125.34, 127.57, 129.73, 135.73, 161.26, 182.17. Melting point 131-133℃. FD-MS: m/z468 (M+).

Step 2: Dissolve compound 2 (37g, 0.079mol) in 200mL anhydrous THF and cool to -78°C. To this solution, n-BuLi (2.5 M in hexanes, 94 mL, 0.24 mol) was slowly added to maintain the temperature below -60 °C. After addition, the orange-yellow solution was stirred at -78°C for 1 hour. Dissolve 4-n-heptylbromobenzene (60.2g, 0.24mol) in 100mL anhydrous THF and add dropwise to the above cooled solution. The reaction was slowly warmed to room temperature and stirred at room temperature overnight. The reaction was quenched with aqueous HI (47% aqueous solution, 143 mL, 0.79 mol) and the TBDMS group was also deprotected. Heat the dark brown reaction to reflux for 10 minutes. Most of the solvent was removed under reduced pressure. The reaction mixture was then extracted three times with dichloromethane. The combined organic phases were washed with saturated sodium metabisulfite solution, water and brine and dried over MgSO4. The crude product was obtained as a brown viscous oil and was purified by silica gel column chromatography using 10/90 ether/hexane as eluent.
The pure product was obtained as 22.4g light yellow-brown solid, with a yield of 51%. 1HNMR (CDCl3) δ (ppm): 0.91 (t, J=7.0Hz, 6H), 1.26-1.43 (m, 8H), 1.71-1.81 (m, 2H), 2.73 (t, J=7.8Hz, 2H) , 5.21 (s, br, 2H, OH), 6.89 (d, J=2.3Hz, 2H), 6.96 (dd, J1=9.5Hz, J2=2.5Hz, 2H), 7.32 (d, J=8.6Hz, 4H, phenyl), 7.35 (d, J=8.6Hz, 4H, phenyl), 7.57 (d, J=9.4Hz, 2H). 13CNMR (CDCl3): 14.16, 22.72, 29.26, 29.52, 3160, 31.86, 35.90, 106.84, 118.58, 127.07, 128.46, 128.98, 129.64, 131.04, 134.94, 136.30, 142.10, 151.69. Melting point 162-164℃. FD-MS: m/z558 (M+).
Main reference materials
[1] EP1289029 Electroluminescent devices having diarylanthracene ladder polymers in emissive layers



 微信扫一扫打赏
微信扫一扫打赏
