Background and overview[1]
5-Methyl-2-(1-methylethoxy)-4-(4-piperidine)-aniline dihydrochloride is a chemical intermediate, also known as (4-(5-iso Propoxy-2-methyl-4-amino-phenyl)-piperidine hydrochloride, CAS number 1380575-45-0, is an intermediate for the preparation of ceritinib. Ceritinib is an oral , anaplastic lymphoma kinase (ALK) inhibitor, which has made breakthrough progress in the treatment of patients with metastatic non-small cell lung cancer (NSCLC) in clinical studies. Approved by the U.S. Food and Drug Administration [FDA] on April 29, 2014 Ceritinib is used for the treatment of patients with anaplastic lymphoma kinase-positive (ALK+) metastatic non-small cell lung cancer (NSCLC) whose disease has worsened after treatment with Xalkori (crizotinib) or who are intolerant to Xalkori.
Preparation[1]
Prepared from 4-(5-isopropoxy-2-methyl-4-nitrophenyl)pyridine in two steps:
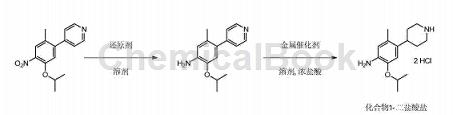
Add 400.0L methanol and 50.0kg 4-(5-isopropoxy-2-methyl-4-nitrophenyl)pyridine into the 1000L hydrogenation reaction kettle. Use vacuum and 0.5MPa argon in the kettle. Replace the gas three times, add 5.0kg of 10% wet palladium on carbon (1.5kg on a dry basis), use vacuum and 0.5MPa hydrogen to replace the kettle three times, maintain the reaction pressure at 0.5MPa, the temperature at 30°C, and react for 5 hours. Filter the reaction solution and store the catalyst temporarily so that it can be reused in subsequent batches. Add the filtrate into a 1000L hydrogenation reaction kettle, add 30.6L concentrated hydrochloric acid, use vacuum and 0.5MPa argon to replace the kettle three times, add 14.5kg 10% wet platinum carbon (dry basis 5.0kg), use vacuum and 0.5MPa hydrogen in the kettle Replace three times, maintain the reaction pressure at 1.2MPa, the temperature at 30°C, and react for 16 hours.
Filtration and temporary storage of catalyst can be repeated in subsequent batches. When the filtrate is below 50°C, concentrate to 100L under reduced pressure. Add 150.0L isopropyl alcohol and concentrate under reduced pressure to 100L below 50°C. Add 150.0L of isopropyl alcohol again, and concentrate under reduced pressure to about 100L below 50°C. Add 100L isopropyl alcohol, stir at 30°C for 3 hours, cool to 0°C, stir for 2 hours, filter, and rinse with isopropyl alcohol. The wet product obtained by filtration was vacuum dried at 55°C for 12 hours to obtain 55.0 kg of off-white solid (dihydrochloride). Yield: 93%, purity 99.7%. After testing, the product was confirmed to be 2-isopropoxy-5-methyl-4-(4-piperidyl)aniline hydrochloride.
Apply[1]
Used to prepare ceritinib, as shown in the figure below:
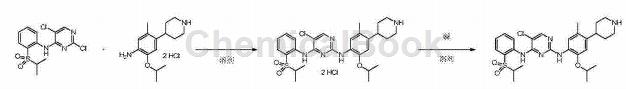
First step: 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonate) Preparation of acyl)phenyl)pyrimidine-2,4-diamine dihydrochloride
Add 80.6kg of isopropyl alcohol, 12.4kg of 5-methyl-2-(1-methylethoxy)-4-(4-piperidine)-aniline dihydrochloride and 14.7kg 2,5-dichloro-N-(2-(isopropylsulfonyl)phenyl)pyrimidin-4-amine, heated to reflux, react for 24 hours. The reaction solution was cooled to 10°C, stirred for 3 hours, and filtered. Add the wet product obtained by filtration into the enamel reaction kettle, add 99.2kg of isopropyl alcohol and 13.0kg of purified water, reflux and stir until the solid is dissolved. Control the temperature to not be lower than 85°C, and add 99.2kg of isopropyl alcohol dropwise. Slowly cool down to 20°C, stir for 2 hours, and filter.
The wet product obtained by filtration will be reused�Add 99.2kg of isopropyl alcohol and 13.0kg of purified water into the enamel reaction kettle, reflux and stir until the solid is dissolved and clear. Control the temperature to not be lower than 85°C, and add 99.2kg of isopropyl alcohol dropwise. Slowly lower the temperature to 20°C, stir for 2 hours, filter, and rinse with isopropyl alcohol. The wet product obtained by filtration was vacuum dried at 55° C. for 16 hours to obtain 16.4 kg of yellow solid (ceritinib dihydrochloride), with a yield of 67% and a purity of 99.9%. The product was tested and confirmed to be 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonyl) Phenyl)pyrimidine-2,4-diamine dihydrochloride. m/z(+ESI)=558.0[M+H]+, 279.7[M/2+H]+.
Second step: 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulfonate) Acyl)phenyl)pyrimidine-2,4-diamine (preparation of ceritinib)
Add 16.4kg of the compound obtained in the previous step and 167.0kg of purified water into a 500L enamel reaction kettle, and stir at room temperature until dissolved. Then add 196.8kg absolute ethanol. Under nitrogen protection, the reaction was heated to 50°C. An aqueous solution of 2.3kg sodium hydroxide and 83.6kg purified water prepared in advance was added dropwise to the reaction solution. After the dripping is completed, the reaction solution is slowly cooled to 20°C and stirred for 2 hours. Filter, and rinse the wet product with a pre-prepared mixed solution of 13.0kg absolute ethanol and 18.0kg purified water. The wet product obtained by filtration was vacuum dried at 50-55°C for 16 hours, and 13.0 kg of off-white solid finished product ceritinib was obtained. Yield 90%, purity 99.8%.
Main reference materials
[1] CN201510897647.3 Preparation method of ceritinib and its intermediates



 微信扫一扫打赏
微信扫一扫打赏
