Background and overview[1][2]
O-ethylphenylhydrazine hydrochloride is an intermediate in the synthesis of the anti-inflammatory and analgesic drug etodolac. In recent years, foreign research on this type of drug has progressed rapidly. Since the mid-1980s, there have been mature patents and various aspects of research are quite sufficient. However, domestic reports are relatively rare. There are experimental synthesis of raw materials used for o-ethylphenylhydrazine. It is o-ethylaniline, which is produced by the reduction of o-nitroethylbenzene, a by-product of chloramphenicol produced by Dongyao Group.
First, o-ethylaniline reacts with sodium nitrite to form o-ethylaniline diazonium salt. The available reducing agents for reducing this diazonium salt to hydrazine include: SnCl2·2H2O, SO2. Among them, the SnCl2·2H2O reduction method is relatively mature and has a high yield, up to 70%. However, SnCl2·2H2O is relatively expensive, the reduction time is long, and the low-temperature reaction must always be maintained. The high cost is not suitable for industrial production.
Apply[3]
O-ethylphenylhydrazine hydrochloride is an intermediate for the synthesis of etodolac, an anti-inflammatory and analgesic drug. In addition, it can also be used as an intermediate for organic synthesis. Examples of its application are as follows: Preparation of 7-ethyl tryptol, 7- Ethyl tryptol is a key intermediate for etodolac, the raw material of non-steroidal anti-inflammatory drugs. The preparation method includes: (1) hydrolyzing 2,3-dihydrofuran in a glycol ether solvent under acidic conditions to obtain 4-hydroxyl Butyraldehyde; (2) 4-hydroxybutyraldehyde and o-ethylphenylhydrazine hydrochloride react in a glycol ether solvent in a temperature range of -20°C – the boiling point of the solvent for 1 to 24 hours, and then post-processed to obtain 7- Ethyl tryptol. The preparation method of 7-ethyl tryptol of the present invention has good yield, convenient operation and high product purity, thereby further preparing etodolac and is suitable for industrial production.
Preparation [1-2]
Method 1: Use o-ethylnitrobenzene, a by-product of the production of p-ethylnitrobenzene, as the raw material, triethylbenzylammonium chloride as the phase transfer catalyst, and use sodium sulfide to reduce it to o-ethylaniline (2 ). Then 2 is diazotized, and o-ethyldiazobenzene is reduced with sodium sulfite to obtain o-ethylphenylhydrazine sulfonate, and hydrochloric acid is added to precipitate 1. Based on o-ethylnitrobenzene, the total yield is 86.4%,
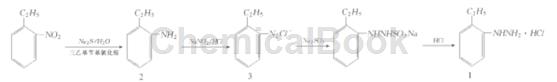
Step 1: Preparation of o-ethylaniline (2)
Add water (300 ml), Na2S (300 g, 3.85 mol), o-ethylnitrobenzene (302 g, 2 mol), and triethylbenzylammonium chloride (9g, 0.039 mol) in sequence. Slowly raise the temperature to reflux, and use GC to track the reaction. The reaction is completed in about 8 hours. Cool to about 50°C and stand to separate the supernatant liquid 2 (232 g, 96%), with a content of 98.2% (Shimadzu GC-14 B gas chromatograph, column C PB1-M25-025, column temperature 160℃, the hydrogen flame detector temperature is 280℃, the injector temperature is 220℃, N2 is the carrier gas, the flow rate is 1 ml/min), and it can be directly used for the next step of synthesis without distillation.
Step 2: Synthesis of o-ethylphenylhydrazine hydrochloride (1)
In a 500 ml three-neck flask, add 31% industrial: hydrochloric acid (147 g, 1.25 mol), water (100 ml), and add 2 (60. 9g 0. 5 mol) dropwise at about 10°C. 30 minutes to complete. Add a solution of sodium nitrite (34.5 g, 0.5 mol) and water (50 ml) dropwise at 0 to 5°C, use starch potassium iodide test paper to detect the end point of the reaction, stir for 10 minutes and set aside. In another 1000 ml four-necked flask, add sodium sulfite (157.5 g, 1.25 mol), water (300 ml) and 31% hydrochloric acid (50 ml). Add the above solution at 85~90°C. Complete the addition in 40 minutes and continue. Reaction 2 h. Add activated carbon (5 g), decolorize for 1 h, and filter. Add hydrochloric acid (130ml) to the filtrate at 95°C and stir for 30 minutes. A large amount of sulfur dioxide gas is released at this time (absorbed with alkali solution). Then cool to 0 ℃, pass
Filter, wash with 10% hydrochloric acid (50 ml×3), and recrystallize with water to obtain white flaky crystals 1 (77.7 g, 89.5%), mp182~183℃ (Literature [1] 181 ~ 183℃). IR and 1 HNMR are consistent with the structural characteristics, and the deviation between the experimental values and calculated values of elemental analysis (C8 H13 ClN2) C, H, and N is less than 0.3%.
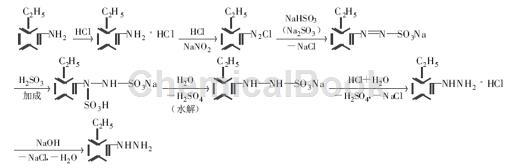
Method 2: Add 18 ml concentrated hydrochloric acid and 18 ml water to the three-necked flask (24 ml concentrated hydrochloric acid and 24 ml water are required when using Na2SO3 as the reducing agent), cool to below 0 ℃ in an ice-salt bath, and slowly drip Add 10.9 g of o-ethylaniline, then add a solution of 6.2 g of sodium nitrite and 14.5 ml of water, use starch KI test paper to check the reaction end point, and use the β-naphthol penetration circle test to check the presence of diazonium salt. The reaction is complete. Then add 37.4 g NaHSO3 and 7.2 g NaOH and 80 ml water (when using Na2SO3 as the reducing agent, add 45.3 g Na2SO3 and 130 ml water), heat to 80 ℃ to form a NaHSO3-Na2SO3 solution, keep the water bath temperature at 75~80 ℃, and dissolve the diazo in about 1 hour After adding the liquid, continue stirring for 2 h to complete the reaction.
Add 47 ml of concentrated hydrochloric acid to the product, let it stand and raise the temperature to 85~90 ℃, then cool down for 5 minutes, and light pink crystals precipitate. After suction filtration, decolorization, hot filtration and recrystallization, white o-ethylbenzene is obtained Hydrazine hydrochloride. Add sodium hydroxide solution to this solid. The upper layer of the solution is an orange-yellow oil layer. After extraction with ether, light yellow flake crystals or orange-yellow oily liquid are obtained, which is o-ethylphenylhydrazine. The yield is 56.4% (using Na2SO3 as 52.5%).
Main reference materials
[1] Synthesis of o-ethylphenylhydrazine by sodium sulfite reduction method
[2] Synthesis of o-ethylphenylhydrazine hydrochloride
[3] CN200510060465.7 Preparation method of 7-ethyl tryptanol



 微信扫一扫打赏
微信扫一扫打赏
