Background and overview[1][2]
2,6-Dichlorofluorobenzene, pure product, is a white crystal with a melting point of 37-40°C and a boiling point of 168-169°C. It is an important intermediate for the synthesis of fluoroquinolones and pesticides. The synthesis of 2,6-dichlorofluorobenzene currently mostly adopts the method of using 2,6-dichloroaniline as raw material through diazotization and thermal decomposition: the process route of this method is mature and the yield is high. However, the existing problems are: (1) The waste gas BF3 produced by diazotization pollutes the air; (2) It is difficult to control the thermal decomposition of diazonium salts, so it is difficult to produce in large quantities; (3) The raw material 2,6-dichloroaniline The price is on the high side. As the market demand for 2,6-dichlorofluorobenzene increases, this method will be difficult to meet this demand, so it is necessary to develop a new synthesis method.
Apply[2-4]
2,6-Dichlorofluorobenzene is an important intermediate in the synthesis of fluoroquinolones and pesticides. Examples of its applications are as follows:
1. Preparation of 2,3,4-trifluoronitrobenzene.
Includes the following steps: (1) In the presence of sulfuric acid, nitration reaction occurs between 2,6-dichlorofluorobenzene and nitric acid. After the reaction is complete, the dichloromonofluoronitrobenzene mixture is obtained through post-treatment; (2) ) Mix the dichloromonofluoronitrobenzene mixture obtained in step (1), potassium fluoride, phase transfer catalyst and 3,5-dichloro-4-fluoronitrobenzene to obtain a mixed system for fluorination reaction, and the reaction is complete Afterwards, after post-treatment, 3,5-dichloro-4-fluoronitrobenzene and the 2,3,4-trifluoronitrobenzene are separated and obtained. In this preparation method, the product obtained through the nitration reaction does not need to be separated, and is directly subjected to the next step of fluorination reaction. In the fluorination reaction, 3,5-dichloro-4-fluoronitrobenzene acts as a Solvent improves the solid-liquid ratio of the reaction and increases the fluidity during the reaction.
2. Preparation of 3,5-dichloro-2,4-difluoroaniline,
This method uses the distillation residue in the production process of 2,4-dichloro-3-fluoronitrobenzene from the nitration of 2,6-dichlorofluorobenzene as raw material, and is prepared by chlorination with chlorine and fluorination with potassium fluoride. 3,5-dichloro-2,4-difluoronitrobenzene; finally reduced to obtain 3,5-dichloro-2,4-difluoroaniline. The present invention uses by-products in the existing production process route as raw materials, and is directly used to synthesize 3,5-dichloro-2,4-difluoroaniline without any treatment, which is similar to the current preparation of 3,5-dichloro-2, Compared with the method of 4-difluoroaniline, it has the characteristics of cheap and easy to obtain raw materials, full utilization of resources, mild reaction conditions, high product yield, and low production cost, and is very suitable for industrial production.
3. Preparation of difluorobenzophenone.
P-Fluorotoluene, chlorine, 2,6-dichlorofluorobenzene, ethanol solution, and benzophenone are used as raw materials. After chlorination reaction, multi-stage cooling and step-by-step crystallization, the target product 4,4′-difluoride is finally obtained. Benzophenone, the purity of this product has been improved and meets the purity requirements for synthetic polyetheretherketone (PEEK). In the preparation process, the raw materials used are easily available, the synthesis process is simple, and the yield of 4,4′-difluorobenzophenone is improved. The invention has broad application prospects and industrial value.
Preparation [1, 5-6]
Method 1: The steps for synthesizing 2,6-dichlorofluorobenzene from 2,3-dichloronitrobenzene are:
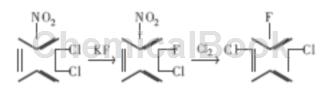
The reaction is carried out in two steps. The first step: 2,3-dichloronitrobenzene undergoes halogen exchange fluorination reaction with KF under the action of solvent or phase transfer catalyst to obtain 2-fluoro-3-chloronitrobenzene. ; Second step: 2-fluoro-3-chloronitrobenzene undergoes a free radical reaction at high temperature, and -Cl replaces -NO2 to obtain the product 2,6-dichlorofluorobenzene.
1) Fluorination reaction
In a dry 250ml three-necked flask, put 57.6g of 2,3-dichloronitrobenzene, 20.88g of dry potassium fluoride, and a certain amount of phase transfer catalyst, and raise the temperature to 175°C with stirring. hour, chromatographic tracking analysis stops the reaction when the product concentration is the highest. Cool to 90 ℃, filter with suction, and wash the filter cake with toluene 2 to 3 times. The filtrate was distilled under reduced pressure, and 42.7g of brown oily liquid containing 91.5% 2-fluoro-3-chloronitrobenzene was isolated, with a yield of 75%.
2) Chlorination reaction
Put 225g of 2-fluoro-3-chloronitrobenzene into a 250ml three-necked flask, heat to increase the temperature, bubble in the dried chlorine gas, keep the reaction temperature at 190 ~ 200 ℃ while passing the chlorine, and carry out reaction distillation. The generated product, 6-dichlorofluorobenzene, is continuously evaporated. After the reaction is over, wash the distillate with dilute alkali solution and water until neutral, and obtain 160g of crude product; the still liquid is combined to continue chlorination next time. Several batches of materials were combined and distilled to obtain pure 2,6-dichlorofluorobenzene, with a capillary chromatographic analysis content of ≥99.0%, a melting point of 39-40°C, and a yield of 80%.
Method 2: Improvement of the preparation method of 2,6-dichlorofluorobenzene, including the following steps:
1) Using 3,5-dichloro-4-fluoroaniline as raw material, react with inorganic acid to obtain a solution containing ammonium salt; the mole of inorganic acid and 3,5-dichloro-4-fluoroaniline The ratio is 1.0~5.0:1, the reaction time is 0.5~1h, and the reaction temperature is room temperature;
2). React the ammonium salt-containing solution obtained in step 1) with the nitrite aqueous solution at a reaction temperature of -5 to 5°C. Terminate the reaction when the reaction product turns the starch potassium iodide test paper blue to obtain a solution containing Solution of diazonium salt; the molar ratio of nitrite to 3,5-dichloro-4-fluoroaniline is 1.0~2.0:1;
3). Add the reducing agent dropwise into the diazonium salt-containing solution obtained in step 2) and react at a temperature of 10 to 70°C for 2 to 5 hours. The reducing agent and 3,5-dichloro-4-fluoroaniline The molar ratio is 1.5~3.0:1;
4). The reaction solution obtained in step 3) is separated, washed with water, and steam distilled to obtain the product 2,6-dichlorofluorobenzene.
Method 3: A method for preparing 2,6-dichlorofluorobenzene. This method first uses 3,5-dichloro-4-fluoronitrobenzene as raw material, and is prepared by iron powder reduction or catalytic hydrogenation. When catalytic hydrogenation is used for 3,5-dichloro-4-fluoroaniline, the conventional process can be used. The reduction using iron powder is as follows:
a. Add 100kg water, 5kg acetic acid (HAc) and 100kg iron powder to the 500L reaction kettle, and add 100kg 3,5-dichloro-4-fluoronitrobenzene in 2 or 3 batches under reflux state. Get grade A;
b. Reflux the product A for 2.5 to 3 hours, and use gas chromatography (GC) to track the peak without raw materials to obtain product B;
c. Cool product B to 40°C to crystallize, rinse with water on the screen to remove fine iron powder, and obtain product C;
d. Dissolve product C with ethanol, filter out the iron powder again to obtain the filtrate, and recover the ethanol to obtain 3,5-dichloro-4-fluoroaniline.
Then in the presence of a reducing agent, 3,5-dichloro-4-fluoroaniline is replaced by diazo hydrogen to obtain 2,6-dichlorofluorobenzene: In a 1000L reaction kettle, add 500L as a reducing agent The aqueous solution of hypophosphorous acid (hypophosphorous acid, also known as hypophosphorous acid, molecular formula H3PO2, the mass percentage of hypophosphorous acid used is 50%) and 3,5-dichloro-4-fluoroaniline (180kg), warm to 40°C, add dropwise Sodium nitrite (NaNO2) solution (90kg solid sodium nitrite dissolved in 200L water), insulate and react for 4 to 5 hours, separate the lower oil phase, neutralize to neutrality, and steam distill to obtain 2,6-dichlorofluoro Benzene 135kg (96.67%, GC), yield 80.56%.
Main reference materials
[1] Research on the synthesis of 2,6-dichlorofluorobenzene
[2] CN201310331193.4 Preparation method of 2,3,4-trifluoronitrobenzene
[3] CN201210050792.43, synthesis method of 5-dichloro-2,4-difluoroaniline
[4] CN201810494105.5 A preparation method of high-purity difluorobenzophenone
[5] CN200910102392.1 A preparation method of 2,6-dichlorofluorobenzene
[6] CN200810303638.7 A preparation method of 2,6-dichlorofluorobenzene



 微信扫一扫打赏
微信扫一扫打赏
