Background and overview[1]
4-Bromo-1-methoxy-2-(3-methoxypropoxy)benzene can be used to prepare aliskiren intermediates.
Aliskiren is a new type of highly effective antihypertensive drug. It is the first drug with a new pharmacological mechanism of action introduced in the field of hypertension treatment. Its mechanism of action is to directly inhibit renin and increase plasma kidney function. Drugs that reduce angiotensinogen activity (PRA) and inhibit the conversion of angiotensinogen into Ang I are often used alone or in combination with other antihypertensive drugs to treat hypertension. It is an orally available, non-peptide renin inhibitor of the renin-angiotensin-aldosterone system (RAS). Because aliskiren has a good clinical effect in treating hypertension, major pharmaceutical companies and research units have attracted widespread attention in recent years to the preparation methods of aliskiren and its important intermediates.
Preparation[1]
In a 50mL round bottom flask, add 2.03g 5-bromo-2-methoxyphenol and 25mL CH3CN and stir to dissolve, then add 3.32g KI and 4.17g K 2CO3 and 1.7mL 1-bromo-3-methoxypropane were heated and refluxed for 24 hours. Steam off CH3CN, extract with Et2O 30mL×4, wash Et2O with 40mL saturated NaCl aqueous solution, MgSO 4dried, filtered, evaporated to dryness, and column separated (CH2Cl2) to obtain 2.66g 4-bromo-1-methoxy-2-( 3-Methoxypropoxy)benzene (97% yield). Reference: Hanessian, S., S.b. Guesné, and E. Chénard, Organic Letters, 2010.12(8):p.1816-1819. NMR data:1H NMR (CDCl3, 600MHz, TMS) δ7.02-7.00(m,2H),6.72(d, J=8.3Hz,1H),4.08(t,J=6.5Hz,2H),3.82(s,3H),3.55(t,J=6.1Hz,2H),3.34(s,3H),2.11-2.07( m,12H).13CNMR (100MHz, CDCl3)δ149.25,148.66, 123.40,116.40,112.95,112.65,69.02,66.21,58.60,56.06, 29.41.
Apply [1]
CN201410166471.X provides a preparation method of aliskiren hydrochloride, including:
1) React a compound with a structure of formula (VII) and a compound with a structure of formula (VIII) to obtain an aliskiren intermediate with a structure of formula (I);
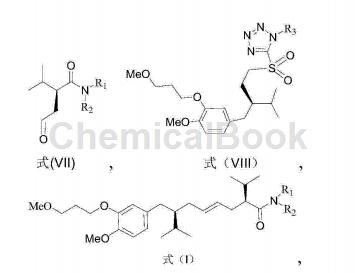
Wherein, R1 and R2 are independently selected from C1 to C12 alkyl groups;
R3 is an aryl group or a C1-C12 alkyl group;
2) React the compound with the structure of formula (I) and N-halosuccinimide under acidic conditions to obtain the compound with the structure of formula (XVI);
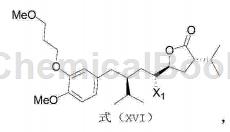
Among them, X1 is Cl, Br or I;
3) Convert the compound with the structure of formula (XVI) into the aliskiren salt with the structure of formula (XVII);
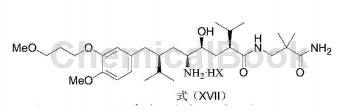
Wherein, the HX is a C1-C8 organic acid or inorganic acid.
4-Bromo-1-methoxy-2-(3-methoxypropoxy)benzene can be used to prepare aliskiren intermediate formula XII:
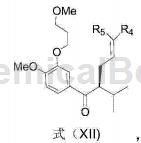
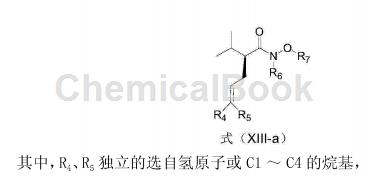
Add 551.1mg 4-bromo-1-methoxy-2-(3-methoxypropoxy)benzene into a dry 50mL round-bottomed flask, and replace the reaction bottle with N2. Add 18 mL anhydrous THF, cool to -78°C, slowly add 1.3 mL n-BuLi THF solution (1.6M) dropwise, and react at -78°C for 3 hours. Then slowly add a THF solution containing 119.9 mg of the compound of formula (XIII-a) dropwise, continue to react at -78°C for 1 hour, then rise to room temperature for 1 hour, and finally quench with 15 mL of saturated NH4Cl aq. Steam off THF, extract with 20mL×4 of CH2Cl2, and use 20mL of saturated NaCl for CH2Cl2 Wash with aqueous solution, dry with MgSO4, filter, evaporate to dryness, and separate on a column (EtOAc: petroleum ether = 1:20) to obtain 198.1 mg of a compound of formula (XII) (yield 96%).

Nuclear magnetic data: 1H NMR (CDCl3, 600MHz, TMS) δ7.56-7.55(m,2H), 6.93 – 6.87(m,1H),5.73-5.66(m,1H),5.02-4.99(m,1H),4.90-4.89(m,1H), 4.18(t,J=6.5Hz,2H), 3.92(s ,3H),3.57(t,J=6.1Hz,2H),3.36(s,3H), 3.30-3.27(m,1H),2.57-2.52 (m,1H), 2.33-2.29(m,1H), 2.14-2.09(m,2H),2.08-2.01(m,1H),0.94(d,J=6.9Hz,3H),0.93 (d,J=6.8Hz,3H).13 C NMR (100MHz, CDCl3)δ202.13,153.44,148.41,136.33, 131.38, 122.66,116.03,112.05,110.18,69.12,65.99,58.58,55.92,51.61,33.34,3 0.63, 29.38,21.15 , 19.54.
Main reference materials
[1] CN201410166471.X Aliskiren intermediate and preparation method of aliskiren
2-4.99(m,1H),4.90-4.89(m,1H), 4.18(t,J=6.5Hz,2H), 3.92(s,3H),3.57(t,J=6.1Hz,2H),3.36 (s,3H), 3.30-3.27(m,1H),2.57-2.52 (m,1H), 2.33-2.29(m,1H),2.14-2.09(m,2H),2.08-2.01(m,1H) ,0.94(d,J=6.9Hz,3H),0.93 (d,J=6.8Hz,3H).13C NMR (100MHz, CDCl3)δ202. 13,153.44,148.41,136.33, 131.38, 122.66,116.03,112.05,110.18,69.12,65.99,58.58,55.92,51.61,33.34,30.63, 29.38,21.15, 19.5 4.
Main reference materials
[1] CN201410166471.X Aliskiren intermediate and preparation method of aliskiren



 微信扫一扫打赏
微信扫一扫打赏
