Overview[1][2]
4-Chloro-3-nitrobenzene is the main raw material for the synthesis of rheumatoid arthritis treatment drug iguratimod (T-164). There is no industrial production in China and it mainly relies on imports. Its traditional synthesis method It is mainly produced from p-nitroanisole as raw material through a five-step reaction of reduction, acetylation, nitration, hydrolysis, and diazochlorination. However, it is not suitable due to problems such as harsh reaction conditions, source of raw materials, and low yield. For industrial production, this article improves the process with reference to other literature, choosing the common and cheap drug paracetamol (acetaminophen) as the starting material, and making it through a four-step reaction of methylation, nitration, hydrolysis, and diazotization and chlorination. It can be seen that this process route has easy sources of raw materials, few steps, and can be industrially produced.
Synthetic route[2][3][4][5]
The synthesis route is shown in the figure below.
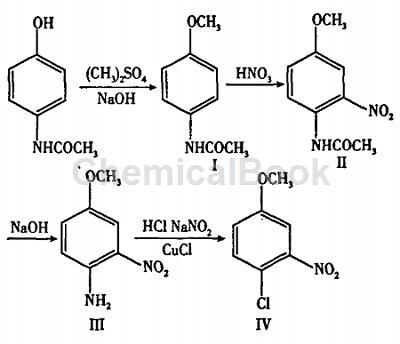
(1) N-(4-methoxy)phenylacetamide (Ⅰ)
Dissolve 30.2 g (0.2 mol) acetaminophen in 400 mL 1 mol/L sodium hydroxide solution, add 38 g dimethyl sulfate (0.3 mol) dropwise, stir at room temperature for 2 hours, and a large amount of solid appears . Filter, and wash the filter cake with water until the washing liquid becomes neutral to obtain 32.8 g of white crystal I, with a yield of 97%, mp127-129°C.
(2)N-(4-methoxy-2-nitro)phenylacetamide (II)
Dissolve 16.5 g of compound I (0.1 mol) in 200 mL of methylene chloride, add dropwise 15 mL of fuming nitric acid, and stir at room temperature for 2 h. Then wash with water, saturated sodium carbonate solution and water in sequence, dry with anhydrous sodium sulfate, rotary evaporate the solvent to obtain a yellow solid, and then recrystallize with 95% ethanol to obtain 19.4 g of yellow needle crystal II, with a yield of 93% , mp118-119℃.
(3) 4-methoxy-2-nitroaniline (III)
Add 10.5 g of compound II (50 mmol) to 100 mL of 4 mol/L sodium hydroxide solution, stir and react at 60°C for 2 h, and a large amount of orange-red solid appeared. Filter, wash the filter cake with water until neutral, and recrystallize with 95% ethanol to obtain 7.7 g of dark red crystal III, with a yield of 91%, mp127-129°C.
(4) 4-Chloro-3-nitroanisole (IV)
After mixing 24.5 g concentrated hydrochloric acid and 24.5 mL water, add 5.5g 4-methoxy-2-nitroaniline (0.03 mol), stir until it dissolves, and then cool down to below 10°C. Add 8 mL of sodium nitrite (2.26 g) solution dropwise. The temperature should not be higher than 5°C. After the dripping is completed, set aside for later use.
Mix 2.26 g cuprous chloride, 6.57 g concentrated hydrochloric acid, and 8.2 mL water, stir to dissolve, then add dropwise the previously prepared solution, control the temperature not to exceed 10°C, and a large amount of foam will be generated. After the dripping is completed, heat to about 45°C, let the gas exhaust, then cool to below 30°C, extract with ethyl acetate, then spin under reduced pressure, and recrystallize the obtained solid with absolute ethanol to obtain 4.2 g of light yellow crystal IV, which is collected The rate is 91%, mp42-44℃.
Method Advantages
The nitration reaction uses fuming nitric acid as the nitrating agent, and fuming nitric acid is used to nitrate it to obtain N-(2-nitro-4-methoxy)phenylacetamide. This avoids the use of mixed acid for nitration, and uses fuming nitric acid. As the nitrating agent, the nitrification yield is the highest. This nitrification method not only saves costs, but also simplifies equipment and operations. In addition, using an aqueous solution of sodium hydroxide as a hydrolysis reagent has a higher yield than using an alcoholic solution of an alkali.
Apply[6]
Iguratimod is a new anti-inflammatory drug that can be used to treat rheumatoid arthritis. 4-Chloro-3-nitrobenzene (compound 2) can be used as a raw material, and under the catalysis of potassium tert-butoxide, it undergoes an etherification reaction with phenol to generate 4-phenoxy-3-nitrobenzene (compound 2). Compound 3); Compound 3 is then reduced by nitro to generate 4-phenoxy-3-aminoanisole (Compound 4); Compound 4 undergoes mesylation reaction with methanesulfonyl chloride in pyridine to generate 4-benzene Oxy-3-methanesulfonamidoanisole (compound 5); then dissolve aluminum trichloride and aminoacetonitrile hydrochloride in nitrobenzene, then add compound 5, and then continuously pass in saturated hydrogen chloride gas Perform Gettleman. Koch reaction produces α-amino-2-methoxy-4-methanesulfonamido-5-phenoxyacetophenone hydrochloride (compound 6); compound 6 is hydrolyzed by methoxy to obtain α-formamide Base-2-methoxy-4-methanesulfonamido-5-phenoxyacetophenone (compound 7); finally compound 7 is obtained by cyclization reaction with N, N-dimethylformamide dimethyl acetal The target product is iguratimod (compound 1).This topic studied the effects of factors such as the feed ratio and reaction time in the etherification reaction; the amount of iron powder and hydrochloric acid used in the reduction reaction; the amount of methanesulfonyl chloride and pyridine used in the mesylation reaction on the product yield; Discussed Gettleman. The synthesis methods and mechanisms of Koch reaction, aminoacylation reaction, methoxy hydrolysis and cyclization reaction, etc. The optimal process conditions were determined: in the etherification reaction, the molar amount of 4-chloro-3-nitrobenzene/phenol/potassium tert-butoxide was 0.25/0.35/0.42, and the reaction was carried out for 5 hours at 110°C, and the yield was 82.5%; in the reduction reaction, each 49% of compound 3 was reacted with 39% reduced iron powder and 1.2mL of 4mol·L -1 hydrochloric acid at 70°C for 1 hour, and the yield was 82. 4%; in 50mL pyridine, 2.59% of compound 4 was reacted with 1.8mL of methanesulfonyl chloride for 1 hour at 0℃-5℃, the yield was 93.0%; the target product iguratimod (compound 1) The overall yield is 37.7%.
References
[1] Wang Yanxiang, Gao Hong, Cao Fenghua. Synthesis study of iguratimod[J]. Chinese Journal of New Drugs, 2006, 15(23): 2042-2044.
[2] MahajanS.NandreR.Studies in the synthesis of 2-meteapto-5-methoxybenzimidazole[J]. Indian Journal ofchemistry, 2006, 45B: 1756-1758.
[3] Gao Xia. Improvement of the synthesis process of 2-nitro-4-methylaniline[J]. Tianjin Chemical Industry, 2006, 20(6): 45-46
[4] Liu Delong, Gao Yan, Gao Yun, etc. Preparation of 2-nitro-4-methoxyaniline
Preparation[J]. Journal of Xuzhou Normal University, 2003, 21(4): 25-27
[5] Qi Gang, Wu Tongxin, Zhang Yinhua. Synthesis of 4-chloro-3-nitrobenzene[J]. Chemical Engineering Times, 2010, 24(9):26-27.
[6] Wei Peipei. Synthesis research of elamod[D]. Nanjing University of Science and Technology, 2009.



 微信扫一扫打赏
微信扫一扫打赏
