Background and overview[1]
4-(4-Fluorophenyl)-1H-imidazole can be used as a pharmaceutical and chemical synthesis intermediate. If 4-(4-fluorophenyl)-1H-imidazole is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing and rinse the skin thoroughly with soap and water. If discomfort occurs, Seek medical attention; if eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Structure
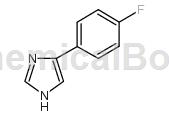
Preparation [1]
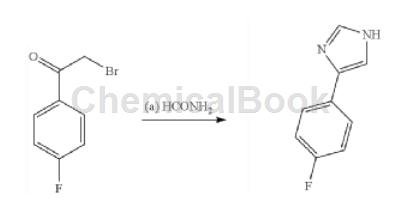
A mixture of 2-bromo-1-(4-fluorophenyl)ethanone (10.9g, 50mmol) and formamide (14mL, 350mmol) was stirred at 170°C under nitrogen for 4 hours. The reaction mixture was cooled and mixed with ethyl acetate (40 mL). Saturated aqueous sodium bicarbonate solution (50 mL) was added carefully while the mixture was cooled in an ice-water bath. Separate the aqueous layer and extract with ethyl acetate (2 × 40 mL). The combined organic solutions were washed with water (30 mL) and brine (30 mL), dried (Na2SO4) and concentrated. Flash chromatography on silica gel (10-100% ethyl acetate in hexanes) afforded 4-(4-fluorophenyl)-1H-imidazole (3.46 g, 21 mmol, 43% yield) as a solid. MS(M+H)+ = 163.1.
Apply[1]
4-(4-Fluorophenyl)-1H-imidazole can be used as an intermediate for pharmaceutical and chemical synthesis. For example, the following intermediate compounds can be prepared:
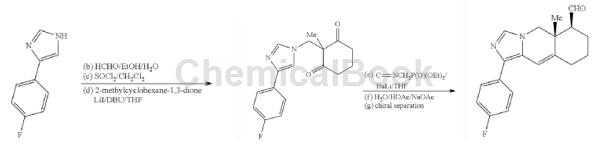
Step 1: To a stirred solution of 4-(4-fluorophenyl)-1H-imidazole (10 g, 62 mmol) in 95% ethanol (25 mL), slowly add aqueous formaldehyde solution (37%, 9 mL). The reaction mixture was stirred at room temperature for 1 hour, then water (25 mL) was added. The reaction mixture was stirred at room temperature for an additional 3 hours. The solid separated from the solution was filtered, washed with aqueous ethanol solution, and dried to obtain (4-(4-fluorophenyl)-1H-imidazol-1-yl) Methanol (11.4g, 59mmol, 96% yield), solid.
Step 2: To a stirred solution of thionyl chloride (18 mL) in anhydrous dichloromethane (180 mL), add (4-(4-fluorophenyl)-1H-imidazol-1-yl)methanol (4-(4-fluorophenyl)-1H-imidazol-1-yl) in portions 11.4g, 59mmol) at room temperature under nitrogen. The reaction mixture was stirred at room temperature for 3.5 hours, then anhydrous toluene (90 mL) was added. The mixture was stirred at room temperature for 30 minutes. Concentrate under reduced pressure to obtain 1-(chloromethyl)-4-(4-fluorophenyl)-1H-imidazole hydrochloride (14.5 g, 59 mmol, 100% yield) as a solid. MS measured value: (M + H) + = 211.3.
Main reference materials
[1] US20110263494. Nonsteroidal compounds useful as modulators of glucocorticoid receptor AP-1 and/or NF-kappab activity and use thereof



 微信扫一扫打赏
微信扫一扫打赏
