Background and overview[1][2]
3-Bromophenylethylamine is mainly used as a pharmaceutical intermediate, such as the synthesis of tetrahydroisoquinolinone compounds. Because tetrahydroisoquinolinone compounds have a variety of biological activities, their synthesis methods and biological activity research have received widespread attention. In addition to being used in the manufacture of highly effective pesticides, color films and dyes, it also has important pharmacological effects, such as vasodilation, anti-tumor, anti-hypertension, anti-arrhythmia, anti-thrombotic activity, etc. Tetrahydroisoquinoline compounds have a greater impact on adrenergic receptors and calcium channels. At the same time, many tetrahydroisoquinoline derivatives themselves are plant bases and have very useful biological activities.
The earliest extracted plant alkaloids were quinine and morphine, and the basic core of some Chinese herbal medicines with cardiovascular pharmacological activity is also tetrahydroisoquinoline. Due to the various pharmacological activities of tetrahydroisoquinoline derivatives, they have played an important role in drug research and pharmaceutical production. 6-Bromo-1,2,3,4-tetrahydroisoquinoline-1-carboxylic acid, as an important tetrahydroisoquinoline derivative, has a large demand especially in cardiovascular diseases, so the synthesis research of this compound is very important. important practical significance.
Preparation [2, 4-5]
Add 300 mL of ethanol, 35.0 g of 3-bromophenylacetonitrile and 5.0 g of radium nickel into a three-necked flask, react at 20°C for 10 hours, filter, and concentrate the filtrate to obtain a crude product, which is recrystallized with petroleum ether to obtain 3-bromobenzene. Ethylamine 34.7g (93.4%).
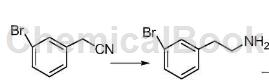
Application
3-Bromophenylethylamine is mainly used as a pharmaceutical intermediate, such as the synthesis of bromo-1,2,3,4-tetrahydroisoquinoline-1-carboxylic acid. Using 3-bromophenylacetonitrile as raw material, 6-bromo-1,2,3,4-tetrahydroisoquinoline-1-carboxylic acid was synthesized through four-step reactions. The synthesis route is as follows:
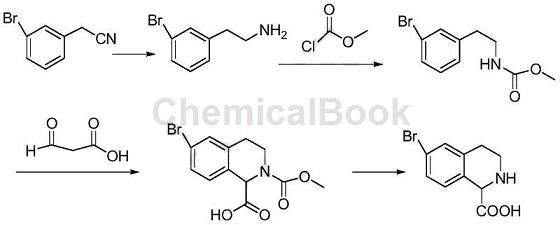
Step 1: Synthesis of 3-bromophenylethylcarbamate methyl ester:
Add 200mL methylene chloride, 32.0g 3-bromophenylethylamine and 17.0g triethylamine into the three-necked flask. At 10°C, add 16.0g methyl chloroformate dropwise. After the dropwise addition is completed, continue the reaction for 2 hours. . Add 300 mL hot water, separate the organic phase, wash with ammonium chloride aqueous solution, wash with saturated brine, dry with anhydrous sodium sulfate, and concentrate to obtain 38.4g (96.7%) of 3-bromophenylethylcarbamate methyl ester.
Step 2: Synthesis of 6-bromo-2-methoxycarbonyl-1,2,3,4-tetrahydroisoquinoline-1-carboxylic acid:
Add 200 mL of tetrahydrofuran, 35.0 g of 3-bromophenylethylcarbamate methyl ester and 50 mL of concentrated sulfuric acid into a three-necked flask, and heat to reflux to react. After the reaction, pour into 500 mL ice water, extract with dichloromethane (150 mL × 3), combine the organic phases, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, and concentrate to obtain 6-bromo-2-methoxycarbonyl-1, 2,3,4-Tetrahydroisoquinoline-1-carboxylic acid 38.9g (91.3%).
Step 3: Synthesis of 6-bromo-1,2,3,4-tetrahydroisoquinoline-1-carboxylic acid:
Add 35.0g of 6-bromo-2-methoxycarbonyl-1,2,3,4-tetrahydroisoquinoline-1-carboxylic acid and 200mL of 10M sulfuric acid into a three-necked flask, and react under reflux for 6 hours. After the reaction, adjust the pH to 7 with sodium bicarbonate, cool down, filter and precipitate the solid, and dry to obtain 21.8g (76.4%) of 6-bromo-1,2,3,4-tetrahydroisoquinoline-1-carboxylic acid.
Main Reference Materials
[1] CN201410065832.1 A chemical synthesis method of 6-bromo-1,2,3,4-tetrahydroisoquinoline-1-carboxylic acid



 微信扫一扫打赏
微信扫一扫打赏
