Background and overview[1][2]
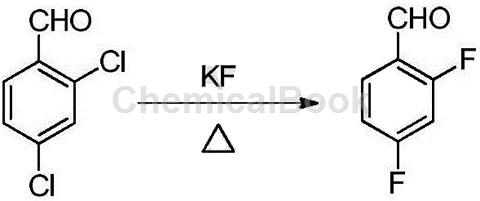
2,4-Difluorobenzaldehyde (DFBA for short) is a widely used organic synthesis intermediate and can be used in the synthesis of medicines, pesticides, dyes and fluorine-containing functional materials. The current methods for synthesizing 2,4-difluorobenzaldehyde mainly include: using 2,4-dichlorobenzaldehyde as raw material and fluorinating it with potassium fluoride to prepare 2,4-difluorobenzaldehyde; Nitrotoluene is used as raw material, and 2,4-difluorotoluene is obtained through reduction, diazotization and fluorination. 2,4-difluorotoluene is chlorinated to obtain 2,4-difluorodichlorobenzyl, which is then hydrolyzed to obtain 2 , 4-difluorobenzaldehyde.
The disadvantage of the above method of synthesizing 2,4-difluorobenzaldehyde is that 2,4-dichlorobenzaldehyde is used as raw material and fluorinated with potassium fluoride to obtain 2,4-difluorobenzaldehyde, which requires a high reaction temperature, because the aldehyde group is unstable at high temperatures and is easily oxidized, so the product is complex, easy to change color, difficult to post-process, the product quality is poor, and due to fluorine exchange, special quaternary phosphorus salts, quaternary ammonium salts or crown ethers are required. When used as a phase transfer catalyst, catalyst recovery is difficult, which also limits its expanded application; when using 2,4-dinitrotoluene as raw material to synthesize 2,4-difluorobenzaldehyde, the synthesis process route is too long and complex, and 2 , 4-difluorotoluene can easily obtain a mixture of monochlorine, dichloride, and trichloride during chlorination. It is usually necessary to control the proportion of dichloride by controlling the depth of chlorination. The separation is difficult, the single-pass conversion rate is low, and the yield Low, high cost.
Apply[3-4]
2,4-Difluorobenzaldehyde, referred to as DFBA, is a widely used organic synthesis intermediate that can be used in the synthesis of medicines, pesticides, dyes and fluorine-containing functional materials. And it is an intermediate for the preparation of a new generation of triazole antifungal drug fluconazole. Examples of its application are as follows:
1. Preparation of 2,4-difluorobenzylamine.
2,4-Difluorobenzylamine is a very important chemical intermediate, and its most important use is in the preparation of the anti-AIDS drug dolutegravir. Dolutegravir is a new type of integrase inhibitor developed by GlaxoSmithKline and approved for marketing in the United States in 2013. Due to its advantages of high efficiency and low toxicity, market sales have increased rapidly in recent years, and the momentum is unanimously optimistic. Among the several currently known synthesis routes for dolutegravir, 2,4-difluorobenzylamine is a key intermediate that cannot be bypassed.
Therefore, it is very meaningful to develop an efficient, green and low-cost preparation process for 2,4-difluorobenzylamine. Its preparation is divided into the following two steps: a). Under a certain pressure, m-difluorobenzene undergoes a carbonylation reaction with CO in the presence of a catalyst to generate 2,4-difluorobenzaldehyde, or make m-difluorobenzene formyl. Chemical reaction: m-difluorobenzene is first substituted with chlorocarbene in a strong alkali system of chloroform, and then hydrolyzed to produce the product 2,4-difluorobenzaldehyde (Reimer-Tiemann reaction). b). The product 2,4-difluorobenzaldehyde in step a) is added to the alcohol solvent, and under a certain pressure in the presence of a catalyst, it directly undergoes a reductive amination reaction with ammonia and hydrogen, or reacts with ammonium formate to prepare 2 , 4-difluorobenzylamine.
2. Synthesis of fenflumidazole.
Fenflumidazole is an important imidazole derivative with anti-inflammatory, analgesic and antipyretic effects. Compared with other NSAIDs, fenflumidazole has better activity and lower side effects, especially gastrointestinal inflammation. In addition, experiments and clinical studies have shown that fenflumidazole also has anti-platelet aggregation effects, can resist platelet adhesion and aggregation, prevent thrombosis, and help prevent atherosclerosis and myocardial infarction.
The preparation method includes: reacting 4-methoxybenzoic acid methyl ester and 4-methoxyacetophenone under the action of a catalyst to generate 1,3-bis(4-methoxybenzene)-1,3 dione, then react with tert-butyl nitrite to generate 1,2-bis(4-methoxybenzene)-1,2dione, and then react with 2,4-difluorobenzaldehyde to generate the 2-( 2,4-Difluorophenyl)-4,5-bis(4-methoxyphenyl)-1H-imidazole.
Preparation [1-2, 4]
Method 1: Using 2,4-dinitrotoluene as raw material, 2,4-difluorobenzaldehyde is prepared through reduction, modified Seeman reaction fluorination, side chain chlorination, and hydrolysis. The total yield is 23.5%, product purity is greater than 99%.
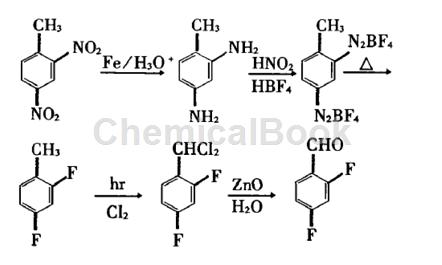
Step 1: Synthesis of 2,4-difluorotoluene
At 250ml In a three-necked reaction flask protected by solid paraffin film, add concentrated hydrochloric acid (40ml), cool to 0°C, add 2,4-diaminotoluene (12.2g, 0.1mol) with stirring, stir and dissolve, and add 40 % of fluoroboric acid (80ml, 0.5mol), stir well, add dropwise a solution of NaNO: (25g, 0.36mol) in water (40ml), control the internal temperature below 0cc, complete the addition in 1 to 2 hours, and continue stirring at low temperature for 0.5 h, use potassium iodide-starch test paper to detect the end point. Excess NaNO: can be removed with sulfamic acid. Suction filtration, the filter cake was washed with cold fluoroboric acid (50ml), ethanol-water solution (1:1), v/v) (50ml), 95% ethanol solution (50ml), ether (50ml×2), and drained Allow to dry, vacuum dry (no heat).
A white solid (18.4g, 75.5%) was obtained. In a fume hood, add the diazonium salt of bisfluoroborate (24.5g, 0.1mol) prepared above into a 50ml round-bottomed flask, connect it to a distillation device and a BF gas absorption device, first use a small fire, and then use a strong fire. , heat the reaction bottle to decompose the bisfluoroboric acid diazonium salt until all the decomposition products are evaporated. The distillate was extracted with methylene chloride, washed with 10% NaOH solution (10ml×2) and water (20ml×2) in sequence, dried with calcium chloride, fractionated under normal pressure, and collected products at 110 ~ 120°C to obtain a colorless liquid ( 5.5g, 42.5%), its refractive index was measured to be 1.4486; content 98.5% (GC).
Step 2: Synthesis of dichloro-2,4-difluorotoluene
A 250ml three-neck bottle is equipped with an air-cooling tube filled with glass rings (glass wool is used in the lower layer), a thermometer and a chlorine pipe. First add 2,4-difluorotoluene (25.6g, 0.2mol) and 0.5g benzyl peroxide. Acyl, heated to 116°C. When reflux occurs, slowly pass in chlorine gas under the irradiation of a 1000-watt iodine tungsten lamp, adjust the heat source to maintain slight reflux, as the color of the solution becomes darker, the boiling point rises, and the weight continues to increase. After 3 hours, the boiling point is 180 ~185℃, weight gain of 15g, no reflux liquid at this time, which is the end point of the reaction. Cool to 10°C, purge nitrogen to remove hydrogen chloride, distill under reduced pressure, and evaporate monochloride at 130°C/40mmHg. The remaining dichloride and trichloride can be directly hydrolyzed without separation.
Step 3: Synthesis of 2,4-difluorobenzaldehyde
Add 100ml of zinc oxide l water into the above dichloride reaction bottle, install a mechanical stirrer and a moisture separator, and use the moisture separator to separate out a milky oil that is heavier than water under rapid stirring and heated reflux. material, the aqueous phase was extracted with 2 × 20 ml of methylene chloride, the organic matter was combined, washed with 10 c of sodium carbonate solution, and dried over anhydrous magnesium sulfate. Recover methylene chloride and collect the product at 82-85°C/40mmHg by distillation under reduced pressure to obtain 24.3g of colorless liquid, with a yield of 85%, a refractive index of 1.4480, and a content of 99.2% (GC).
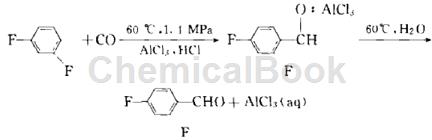
Method 2: Mix 57 g 1,3-difluorobenzene (0.5 mol) and 66.7 g AlCl3 (0.5 mol) into a slurry and add it to the autoclave. Add 5 drops of concentrated HCl, seal the lid of the autoclave, and pass N2 into the autoclave to drive out the air. When the temperature of the heated mixture rises to 60°C, start to pass CO and purge with CO. Three times, the system pressure was raised to 1.5 MPa during each purge. After the third purge, CO was introduced under a pressure of 1.5 MPa and maintained. The reaction continued under this pressure for about 20 h.
After the reaction is completed, the temperature is cooled. The resulting mixture is poured into about 300 ml of ice water, and then 300 ml of cyclohexane is added. Use a separatory funnel to remove the upper organic layer and wash it with water three times, and then dry it over anhydrous magnesium sulfate. .The water layer is subjected to AlCl3 recovery treatment; the dried organic phase is vacuum distilled to recover cyclohexane and unreacted 1,3-difluorobenzene, and continues to be distilled to obtain 2,4-difluorobenzaldehyde. 47.5 g, yield 66.9%. Gas chromatography analysis of 2,4-difluorobenzaldehyde is ≥98.81%.
Method 3: A method of continuously oxidizing 2,4-difluorotoluene to prepare 2,4-difluorobenzaldehyde using a tubular reactor with a special structure, following the following steps:
(1) First, stir and mix the substrate 2,4-difluorotoluene and part of the carboxylic acid solvent at a volume ratio of 1:1 at room temperature, and mix the oxidant and part of the carboxylic acid solvent at a volume ratio of 1:1. Evenly, then mix the metal complex and pour it into the 2,4-difluorotoluene-carboxylic acid solution, and pour the sodium salt into the hydrogen peroxide-carboxylic acid solution; through the required reaction time, calculate the difference between the two materials The flow rate is continuously pumped into the tubular reactor through a metering pump, and then enters the reaction zone for reaction after preheating and mixing. The reaction temperature is controlled by an external circulation heat exchange system;
(2) Control the molar ratio of the reaction materials by adjusting the flow rate and weighting, and control the residence time of the material mixing reaction from 60 to 1800s by changing the inner diameter of the pipe of the tubular reactor from 0.5 to 15 mm and the volume from 25 to 750 ml; After the reaction is completed, the product flows out from the end of the reactor and enters the collection tank. The product is distilled and separated. The unreacted 2,4-difluorobenzaldehyde is recycled and reacted. The product 2,4-difluorobenzaldehyde is collected after distillation and purification. The target The yield of the product 2,4-difluorobenzaldehyde can reach 20% to 35%.
Main reference materials
[1] Synthesis of 2,4-difluorobenzaldehyde
[2] CN201810790781.7 A new synthesis method of 2,4-difluorobenzylamine, the key intermediate of dolutegravir
[3] CN201210078519.2 A method of synthesizing fenflumidazole
[4] CN201610971995.5 A method for preparing 2,4-difluorobenzaldehyde by continuous oxidation of 2,4-difluorotoluene
The internal circulation heat exchange system is controlled;
(2) Control the molar ratio of the reaction materials by adjusting the flow rate and weighting, and control the residence time of the material mixing reaction from 60 to 1800s by changing the inner diameter of the pipe of the tubular reactor from 0.5 to 15 mm and the volume from 25 to 750 ml; After the reaction is completed, the product flows out from the end of the reactor and enters the collection tank. The product is distilled and separated. The unreacted 2,4-difluorobenzaldehyde is recycled and reacted. The product 2,4-difluorobenzaldehyde is collected after distillation and purification. The target The yield of the product 2,4-difluorobenzaldehyde can reach 20% to 35%.

Main reference materials
[1] Synthesis of 2,4-difluorobenzaldehyde
[2] CN201810790781.7 A new synthesis method of 2,4-difluorobenzylamine, the key intermediate of dolutegravir
[3] CN201210078519.2 A method of synthesizing fenflumidazole
[4] CN201610971995.5 A method for preparing 2,4-difluorobenzaldehyde by continuous oxidation of 2,4-difluorotoluene



 微信扫一扫打赏
微信扫一扫打赏
