Background and overview[1][2]
2,3,4,5-Tetrahydro-1H-benzo[d]azepine (hydrochloride), CAS number 4424-20-8, chemical formula C10H13N. Molecular weight 147.21700. Density 0.981g/cm3, boiling point 258.8ºC at 760 mmHg, flash point 114.9ºC, refractive index 1.53. 2,3,4,5-Tetrahydro-1H-benzo[d]azepine (hydrochloride) is available Used as pharmaceutical and chemical synthesis intermediates. If 2,3,4,5-tetrahydro-1H-benzo[d]azepine (hydrochloride) is inhaled, move the patient to fresh air; in case of skin contact, remove contaminated clothing and use Rinse skin thoroughly with soap and water. If you feel discomfort, seek medical attention. If eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately. If ingested, rinse mouth immediately. Do not induce vomiting and seek medical attention immediately. .
Structure
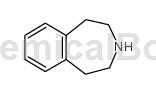
Preparation [1]
The synthesis method of 2,3,4,5-tetrahydro-1H-benzo[d]azepine is as follows:
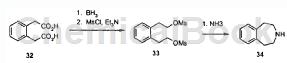
Step 1: Dissolve (2-carboxymethyl-phenyl)-acetic acid (5.0g/25mmol) in THF (100mL) and cool to 0°C. Then borane (1M solution in THF, 100mL/100mmol) was added dropwise while stirring (hydrogen evolution). The reaction was stirred at 0 °C for 4 h and then carefully quenched by adding water (approximately 20 mL). The reaction was partitioned between diethyl ether (200 mL) and water (200 mL). The organic layer was extracted with an additional portion of diethyl ether (100 mL) and the combined organics were washed with brine (100 mL), dried over magnesium sulfate, filtered and concentrated. 2-[2-(2-Hydroxy-ethyl)-phenyl]-ethanol (3.3 g/80%) was obtained.
Step 2: Dissolve 2-[2-(2-hydroxy)-ethyl)-phenyl]-ethanol in dichloromethane (100 mL) and cool to 0°C. Add triethylamine (11.0 mL, 80 mmol), followed by methanesulfonyl chloride (3.5 mL/44 mmol, exothermic) dropwise. After 30 minutes, pour the reaction into a separatory funnel and wash with 1N hydrochloric acid (100mL), water (100mL) and sodium bicarbonate aqueous solution (100mL). The organic solution was then dried over magnesium sulfate, filtered and concentrated to obtain 2-[2-(2-methanesulfonyloxy-ethyl)-phenyl]-ethyl methanesulfonate as a yellow oil (6.0g/quant. ), solidify after standing.
Step 3: Add 2-[2-(2-methanesulfonyloxy-ethyl)-phenyl]-ethyl methanesulfonate (3.2g/10mmol) in acetonitrile and hydroxide to a 350mL pressure vessel 1:1 mixture of ammonium (total volume 100 mL). The container is sealed and heated to 100°C for 1 hour (pressure rises to approximately 40 psi), then gradually cooled. The reaction contents were poured into water and acidified to approximately 50°C. Adjust pH 4 with concentrated hydrochloric acid. The aqueous solution was extracted with diethyl ether (100 mL), basified to pH 14 with 30% aqueous sodium hydroxide solution, and finally extracted repeatedly with 10% methanol/dichloromethane (4 × 50 mL).
The combined organic extracts were dried over magnesium sulfate, filtered and concentrated to obtain 2,3,4,5-tetrahydro-1H-benzo[d]azepine as a light yellow oil (1.2g/81 %), solidify after standing. It forms a salt with hydrochloric acid to obtain 2,3,4,5-tetrahydro-1H-benzo[d]azepine (hydrochloride).
Main reference materials
[1] WO2008051547.FUSED BICYCLIC DERIVATIVES OF 2,4-DIAMINOPYRIMIDINE AS ALK AND C-MET INHIBITORS



 微信扫一扫打赏
微信扫一扫打赏
