Background and overview[1][2]
2-Bromo-4-methoxyaniline is an important pharmaceutical intermediate and can be used as an important intermediate for anti-cancer drug AZD9291 and cerebral circulation, anti-asthma and anti-ulcer drugs. 6-hydroxy-2(1H) – Quinolinones and their derivatives.
Preparation[1]

Yuan Jiacheng et al. reported the use of 1-butyl-3-methylimidazole tribromide ([ bmim] Br3) as the brominating agent to synthesize 2-bromo-4-methoxy aniline.
1. Preparation of ionic liquid [bmim] Br3
Add 0.3 mol of 1-methylimidazole and 90 mL of toluene into a 250 mL three-necked flask, add 0.3 mol of 1-bromobutane dropwise while stirring, and heat to reflux for 12 h. Cool, separate the toluene layer, wash with ethyl acetate and chloroform, and dry under reduced pressure at 80°C for 48 hours to obtain ionic liquid 1-butyl-3-methylimidazole bromide ([bmim] Br). Add 0.3 mol of bromine dropwise to the above ionic liquid, and stir for 2 hours at room temperature. Wash twice with 60 mL of ethyl acetate and dry under reduced pressure to obtain ionic liquid 1-butyl-3-methylimidazole tribromide ([ bmim] Br3).
Synthesis of 2. 2-bromo-4-methoxyaniline
Add 20 mmol p-methoxyaniline (PMOA) and 20 mmol ionic liquid [bmim] Br3 into the three-necked flask, and stir for 30 minutes at 20°C. After the reaction is completed, extract with diethyl ether, evaporate the diethyl ether, and recrystallize the residue with dichloromethane and dry to obtain 3.97 g of 2-bromo-4-methoxyaniline.
2-Bromo-4-methoxyaniline, white solid, yield 98.2%, melting point 62~63℃. 1 HNMR (CDCl3)δ:3.72(s, 3H), 4.96 (s, 2H), 6.21~6.68 (m,3H); IR (KBr), ν/cm -1: 3412, 1602, 1513, 1458, 1231, 582. The IR spectrum of 2-bromo-4-methoxyaniline is shown in Figure 1, and the 1 HNMR spectrum is shown in Figure 2.
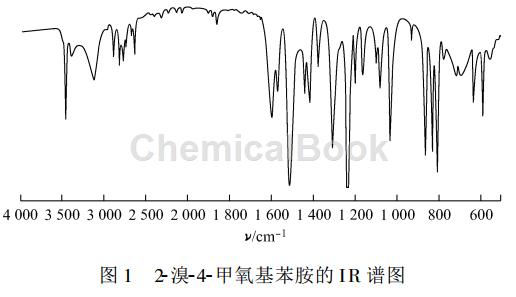
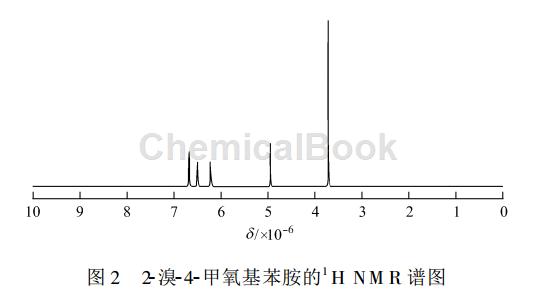
Apply [2]
For preparation of AZD9291:
AZD-9291 is a drug for the treatment of small cell lung cancer with EGFR mutations. The efficacy of AZD-9291 treatment in the T790M mutation population was 66%. Mutation-positive patients who received AZD-9291 had an overall disease control rate of 94%. AZD-9291 has been granted “Breakthrough Therapy” designation by the U.S. Food and Drug Administration (FDA). 2-Bromo-4-methoxyaniline can be used as a starting material to prepare compound AZD9291 and its key intermediates. The synthesis route is as follows:
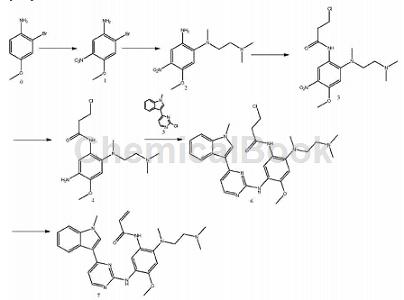
Includes the following steps:
(1) Add 2-bromo-4-methoxyaniline to concentrated sulfuric acid, add potassium nitrate in batches, perform nitration reaction, and obtain compound 1 after post-treatment; (2) Dissolve compound 1 in tetrahydrofuran to prepare Grignard reagent I, Add trimethylethylenediamine and perform Grignard reaction to obtain compound 2; (3) Compound 2 is dissolved in solvent Ⅰ, add 3-chloropropionyl chloride dropwise, and acylation reaction is performed to obtain compound 3; (4) Compound 3 is dissolved in solvent Ⅱ , reduction reaction to obtain compound 4; (5) Dissolve compound 5 in tetrahydrofuran to prepare Grignard reagent II, add compound 4, Grignard reaction to obtain compound 6; (6) Compound 6 is eliminated under the action of a catalyst to obtain compound 7. This method has the advantages of wide sources of raw materials, low cost, simple operation, recyclable solvents, small waste liquid discharge, high product recovery rate, etc., and is easy to realize industrialization.
Main reference materials
[1] Yuan Jiacheng, Ding Yufei. Research on the synthesis of 2-bromo-4-methoxyaniline in ionic liquids [J]. Chemistry World, 2011, 52(12):747-750.
[2] CN201810227822.1 Preparation method of anti-tumor drug AZD9291



 微信扫一扫打赏
微信扫一扫打赏
