Background and overview[1][2]
2-Nitroresorcin is not only an important industrial raw material, but also one of the key synthetic intermediates because the nitro group can be easily reduced to the amino group. It is used because the phenolic hydroxyl group helps to improve the stability of the image. Used as a stabilizer for color photo developers; it can be used to synthesize crown ethers as π-receptor macrocyclic compounds, and to synthesize 2-pyridone derivatives as HIV-1-variant reverse transcriptase inhibitors.
At present, the main methods for preparing 2-nitroresorcin are: Method 1, using resorcin as raw material and directly nitrating it with mixed acid. Due to the high reactivity of the phenolic hydroxyl group and the large number of by-products, the yield is only 12.9 %; Method 2, using resorcinol as raw material, first sulfonating the 4 and 6 positions with sulfocarboxyl groups, then nitrating it with a mixed acid composed of nitric acid and sulfuric acid, and then steam distilling the product to obtain a yield of 37.5%
Apply[2-3]
2-Nitroresorcin is not only an important industrial raw material, but also one of the key synthetic intermediates because the nitro group can be easily reduced to the amino group, and has a wide range of applications. The main applications are: 2-nitroresorcin appears yellow in acid and red in alkali. The color change pH=5.4~7.4, pKIn=6.4±0.1, pT=6.2, because its pK is close to that of strong acid and strong acid The isoelectric point of alkali (pI=7.0), so it can be used as an acid-base indicator; 2-nitroresorcinol and its salts are used as coupling agents for the synthesis of oxidative hair dyes and for polyesters, silk wool Acid azo dyes for similar and blended textiles; used as a stabilizer for color photo developers because the phenolic hydroxyl group helps to improve image stability; can be used to synthesize crown ethers as macrocyclic compounds that serve as π-acceptors. 2-pyridone derivatives serve as HIV-1-variant reverse transcriptase inhibitors, etc.
If some research has developed a preparation method for benzoxazine agonists, the target compound is synthesized using 2-nitroresorcinol as a raw material and after a seven-step reaction. The synthesis method is simple to operate, avoids the use of toxic reagents, and can be used to prepare larger amounts of BI-167107 and similar compounds.
Preparation[1-2]
Method 1: Use a mixed acid composed of concentrated nitric acid, glacial acetic acid and concentrated sulfuric acid as the directional nitrifying agent. The average total product yield is 48.27%. The reaction principle is shown in the figure.
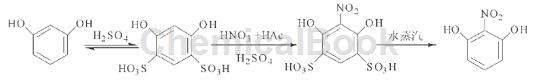
Step 1: Sulfonation. Add 22.00g (0.2mol) of finely ground resorcinol and 60.00g (0.6mol) of concentrated sulfuric acid to a 250mL three-necked flask equipped with a thermometer and mechanical stirring. Stir fully and slowly heat the water bath to 60 to 65°C and keep warm. After reacting for 3 hours, cool naturally to room temperature and keep it airtight for 3 hours. The sulfonated product will be directly used in the next reaction without separation.
Step 2: Nitrification. Install a thermometer, mechanical stirring, and dropping funnel into the three-necked flask of the previous reaction and place it in an ice-water bath. When the temperature in the flask drops to 5°C, slowly add 52.00 g of pre-cooled mixed acid (containing 30.28 mol of HNO) dropwise while stirring. , w(HNO3): w(HAc): w(H2SO4)=1 g: 0.5 g: 0.5 g) nitrification, maintain the temperature at 5 ~ 10 ° C; after the dripping is completed (about 2 h), keep the reaction for 2 h and then Add 80 g of crushed ice and 40.00 g of urea, and continue stirring for 1 h.
Step 3: Purification. Transfer the mixture in the three-necked flask in the previous step into a 500mL flask and perform steam distillation. Collect the fractions on the wall of the condensation tube and in the receiving flask to obtain 14.96 g of orange-red solid 2-nitroresorcinol, with a yield of 48.26 %.
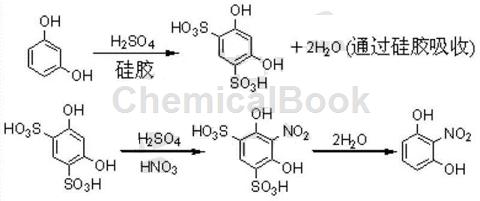
Method 2: A method of synthesizing 2-nitroresorcinol using silica gel as a dehydration aid, including the following steps:
1) According to the mass of resorcin: volume of 98% concentrated sulfuric acid: volume of 65% concentrated nitric acid, measure 98% concentrated sulfuric acid and 65% concentrated nitric acid in a ratio of 10:3~7:6~10, and add 98 % concentrated sulfuric acid and 65% concentrated nitric acid are mixed to obtain sulfur-nitric mixed acid. After sealing, use an ice-water bath to cool it to below 5℃;
2) Mix resorcinol and silica gel at a mass ratio of 5 to 20:1 and put them into the reactor, then slowly drip them at a ratio of resorcinol mass:98% concentrated sulfuric acid volume of 1:1.6 Add 98% concentrated sulfuric acid, and after the dropwise addition is completed, turn on the mixer; and under stirring, continue to dropwise add 98% concentrated sulfuric acid at a ratio of resorcin mass: 98% concentrated sulfuric acid volume of 1:3.4; after the dropwise addition is completed, add 98% concentrated sulfuric acid. Slowly heat to 30-65°C with full stirring, keep warm for 15-60 minutes, and then use an ice-water bath to cool the reactant to below 5°C; then add the cooled sulfur-nitric mixed acid prepared in step (1) dropwise, while maintaining ReverseThe temperature is 5°C; after the dropwise addition is completed, keep it at 5°C for 2 to 3.5 hours, then add crushed ice and urea into the reactor at a mass ratio of resorcin: ice: urea of 1:4:0.02, and continue stirring. Until completely dissolved;
3) Steam distill the mixture obtained in step (2) and collect the fractions. Cool with ice water and then suction filter. Add the solid obtained by suction filtration to 50% ethanol and heat under reflux to dissolve. Resorcinol mass: The volume of 50% ethanol is 1:4; after the solid is completely dissolved, take it out and cool it with ice water, and all 2-nitroresorcinol will precipitate; after suction filtration, wash with 50% ethanol solution, drain and dry. The final product of 2-nitroresorcinol is obtained. The specific reaction equation is:
Main reference materials
[1] Optimization of the preparation process of 2-nitroresorcinol
[2] CN201410095991.6 Method for synthesizing 2-nitroresorcinol using silica gel as dehydration aid
[3] CN201210512530.5 Preparation method of benzoxazine agonist



 微信扫一扫打赏
微信扫一扫打赏
