Background and overview[1][2]
2,6-Difluorobenzoyl chloride can be used as an intermediate for pharmaceutical and chemical synthesis. If 2,6-difluorobenzoyl chloride is inhaled, move the patient to fresh air; if the skin comes into contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable; if the eye contact If exposed to sunlight, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Apply[1-4]
2,6-Difluorobenzoyl chloride can be used as an intermediate for pharmaceutical and chemical synthesis. Examples of its application are as follows:
1. Prepare flufenuron.
The preparation process includes the following steps: (1) Using 3,5-dichloro-4-(1,1,2,2-tetrafluoroethoxy)aniline and sodium cyanate as raw materials, in an acetic acid aqueous solution, Add a phase transfer catalyst and react at 0-80°C for 1-12 hours. The reaction solution is filtered and the filter cake is washed and dried to obtain 3,5-dichloro-4-(1,1,2,2-tetrafluoroethoxy). Phenylurea; (2) In an organic solvent, a mixture of anhydrous zinc chloride and anhydrous aluminum trichloride is used as a catalyst, and 3,5-dichloro-4-(1,1,2,2-tetrafluoro Ethoxy)phenylurea and 2,6-difluorobenzoyl chloride are used as raw materials and reacted at 65-150°C for 1-24 hours. During the reaction, vacuum is used to remove the hydrogen chloride gas. At the end of the reaction, the solvent is recovered by distillation under reduced pressure. After cooling, Add 5% mass fraction of sodium bicarbonate solution, adjust the pH to 7-8, and stir for 30 minutes. The reaction solution is filtered to obtain a filter cake that is washed, dried, and recrystallized to obtain flubenzuron. The process is simple and reasonable, has high safety, few side reactions, easy product purification, high reaction yield and product purity, and has great industrialization potential and social and economic benefits.
2. Preparation of carboxyl-containing bisfluoromonomer.
Specific steps: Prepare 2 under an argon atmosphere using 2,6-difluorobenzoic acid as raw material, dichlorosulfoxide as both solvent and reactant, and N,N-dimethylformamide as catalyst. , 6-difluorobenzoyl chloride; then under argon atmosphere and anhydrous conditions, use anhydrous aluminum trichloride, 2,6-difluorobenzoyl chloride and 3-phenylpropionic acid as raw materials to prepare a white Crystalline bisfluoromonomer, 3-[4-(2,6-difluorobenzoyl)phenyl]propionic acid. The carboxyl-containing polyaryl ether prepared and synthesized by the present invention introduces ionizable polar groups compared to ordinary polyaryl ether polymers, and can be used in membrane separation technology. Since it has negatively charged carboxyl groups, it can be used for water separation. The hydrophilic properties and anti-fouling properties of the membrane are improved, and the conductivity of the proton exchange membrane is improved.
3. Used in the field of functional polymer materials, specifically involving a series of electroactive polyarylene ether polymers and preparation methods of this series of polymers.
It is obtained by acylation reaction between the reduced parent aniline tetramer and 2,6-difluorobenzoyl chloride to obtain the bisfluoromonomer containing the aniline segment, and then the bisfluoromonomer and bishalogen are substituted. The terpolymerization of benzophenone or benzyl sulfone and bisphenol monomer is carried out. By adjusting the feeding ratio of the two bishalogen substituted monomers, a series of side chain polyaryl ether copolymers with different aniline segment contents are obtained. The aniline oligomer chain segment synthesized by this method serves as a side chain polyarylene ether polymer with obvious electrical activity and good thermal stability, and has good solubility in common organic solvents and high processability. It can be used in anti-corrosion coatings. It has broad application prospects in layer materials, packaging materials, etc.
4. Preparation of N-tert-butyl-N’-fluorobenzoyl-substituted pyridinecarboxylic hydrazide derivatives.
N-tert-butyl-N, N’-bishydrazide compounds are used as insect growth regulators to control agricultural pests by simulating the process of 20-hydroxyecdysone stimulating insect ecdysone receptors. Because this type of compound is highly selective against pests such as Lepidoptera, is safe for non-target organisms such as mammals and fish, and is environmentally friendly, it is known as the “third generation pesticide”.
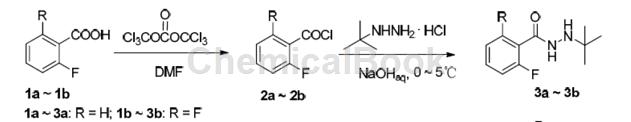
Its synthesis can use 2,6-difluorobenzoic acid as raw material, chlorinated form 2,6-difluorobenzoyl chloride, and the synthetic intermediate N-tert-butyl-N’-fluoro-substituted benzoyl hydrazide In the process of 3a~3b, a slight excess of sodium hydroxide and tert-butylhydrazine hydrochloride was used to prevent N-tert-butyl-N’-substituted benzoyl hydrazide 3a~3b from fluorine-substituted benzoyl chloride 2a ~2b further reacts to form a bis-acylated impurity. In addition, since the fluorine atom is an electron-withdrawing group, benzoyl chloride containing two fluorines at the ortho position of the benzene ring connected to the acyl group is more active than benzoyl chloride containing one fluorine, and tert-butylhydrazine is more likely to interact with 2, 6-Difluorobenzoyl chloride (2b) undergoes nucleophilic substitution reaction, so the yield of 3b is slightly higher than that of 3a.
Preparation [5]
Step 1: In a 250 ml three-necked flask equipped with a reflux condensation device, add 2,6-difluorobenzoic acid, anhydrous KF, DMF and a small amount of catalyst in sequence, heat to reflux, react for 8 hours, cool and filter. Wash the filter residue with DMF, combine the filtrate and washing liquid, evaporate the DMF under reduced pressure, wash with water, and filter to obtain the intermediate product 2,6-difluorobenzoic acid as a light yellow solid.
Step 2: In a 250 ml dry three-necked flask equipped with a reflux condensation device, add 2,6-difluorobenzoic acid, thionyl chloride and a small amount of catalyst in sequence, heat to reflux, react for 4 hours, and evaporate excess Thionyl chloride was used to obtain the product 2,6-difluorobenzoyl chloride, a brown liquid.
Main reference materials
[1] CN201310129728.X Preparation method of flufenuron
[2] CN201510345369.0 Carboxyl group-containing bisfluoromonomer, preparation method and application in preparing carboxyl group-containing polyarylene ether
[3] CN201010293748.7 Side chain electroactive polyarylene ether polymer and its preparation method
[4] Synthesis and insecticidal activity of N-tert-butyl-N’-fluorobenzoyl-substituted picolinate carboxyl hydrazide derivatives
[5] Qin Wei. Design and synthesis of thrombin inhibitors[D]. Central South University, 2009.



 微信扫一扫打赏
微信扫一扫打赏
