Background and Overview
3′-Fluoroacetophenone, also known as m-fluoroacetophenone, is a commonly used drug synthesis intermediate,
Preparation[1-2]
Method 1,
Catalyst [(IPr)AuCl] (3.1mg, 0.5mol%), 3-fluorophenylacetylene (1mmol), methanol (1ml) and water (0.5ml) were added to the 25ml reactor in sequence. The reaction mixture was reacted at 110°C for 6 hours and then cooled to room temperature. The solvent was removed by rotary evaporation, and then the pure target compound was obtained through column chromatography (developing solvent: petroleum ether/ethyl acetate), yield: 97%. 1HNMR(500MHz, CDCl3)δ7.74(d,J=7.10Hz,1H,ArH), 7.63(d,J=8.90Hz,1H,ArH ),7.45(q,J=7.10Hz,1H,ArH),7.30-7.24(m,1H,ArH),2.60 (d,J=0.85Hz,3H,CH3);13CNMR (125MHz, CDCl3) δ196.47,162.62(d,JC-F=245.65Hz),138.99(d,JC-F=5.8Hz),130.55(d,JC-F=7.47Hz),123.94,119.84(d,JC -F=22.00Hz),114.63(d,JC-F=22.63Hz),26.35
Method 2,
Zhao Haoyu reported that after using m-bromofluorobenzene (2) and magnesium wire to prepare Grignard reagent, 3′-fluoroacetophenone, namely compound 1 (Figure 1), was obtained through acetylation and hydrolysis. Conditions such as ratio, solvent dosage, reaction time and temperature were optimized. When preparing Grignard reagent, adding a little iodine first can significantly shorten the reaction induction period; the dropwise addition and total reaction time of 2 should be controlled at 4h and 6h respectively, because although extending the reaction time is beneficial to improving the conversion rate of raw materials, it is easy to form benzene rings. of dimer by-products. Experiments show that the amount of solvent is closely related to the yield. When the concentration of 2 in THF is lower than 2.1 mol/L, the conversion rate is higher. The optimal conditions determined are: using THF as the solvent, the feed ratio is 2: magnesium wire: acetic anhydride = 1:1:1.1 (molar ratio), react at 40~45°C for 4 hours, then raise the temperature to 55~65°C before reacting 2h, acetylation at 0~5℃ for 30min, the total reaction yield is 55%, and the purity is 99.1% (GC method).
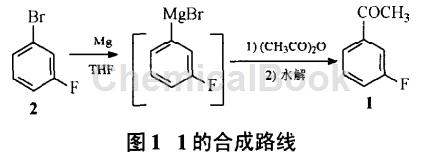
Example: Add THF (300ml) and magnesium wire (85.5g) to a dry four-necked flask, add 2 (8.8g, 0.05mol) dropwise, add a little iodine while stirring to initiate the reaction, control the temperature at 40~45°C and then Add a solution of 2 (604g, 3.45mol) in THF (1.4L) and complete the dripping in about 4 hours. Then react at 55-65°C for 2 hours to obtain a Grignard reagent solution.
A solution of acetic anhydride (393g, 3.85mol) in THF (350ml) was added dropwise to the Grignard reagent obtained above at 0°C, and the reaction was completed at 0~5°C for 30 minutes. Add water (500ml) below 20℃ and stir until the liquid is clear. The mixture was allowed to stand for separation, and THF was evaporated from the organic phase under normal pressure, and then the remaining THF was washed away with water. The 81°C/1.2kPa fraction of the residue was collected under reduced pressure to obtain light yellow liquid 1 (266.5g, 55.2%), with a purity of 99.1% (GC method ).
Main reference materials
[1] CN201510103316.8 A method for synthesizing ketones by hydrolyzing alkynes
[2] Zhao Haoyu. Preparation of m-fluoroacetophenone[J]. Chinese Journal of Pharmaceutical Industry, 2005(02):11.



 微信扫一扫打赏
微信扫一扫打赏
