Overview
P-Methoxybenzoic acid, also known as 4-anisic acid and anisic acid, is colorless needle-shaped crystals at room temperature. It is soluble in ethanol, ether, and chloroform, slightly soluble in hot water, and insoluble in cold water. It is an important pharmaceutical intermediate and can also be used in flavors and preservatives. Therefore, it has relatively good market prospects. However, p-methoxybenzoic acid is generally produced using intermittent kettle reactions in the traditional production process, which has a series of disadvantages such as cumbersome operations, low production efficiency, low raw material utilization, and large amounts of three wastes. As the national environmental protection situation becomes increasingly severe, the elimination of backward production capacity has become an inevitable trend, and the production model of paramethoxybenzoic acid also needs to be improved urgently.
1 Synthesis principles and methods
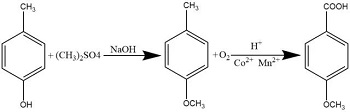
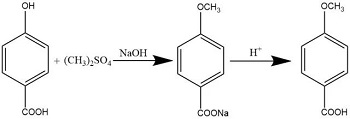
There are two main methods for synthesizing p-methoxybenzoic acid. One is to use p-cresol as raw material. p-cresol first reacts with dimethyl sulfate to p-methyl anisole, and then uses a strong oxidizing agent to The methyl group is oxidized to a carboxyl group to obtain the product p-methoxybenzoic acid. The operation of this method is relatively cumbersome and post-processing is difficult. Another method is to methylate p-hydroxybenzoic acid with dimethyl sulfate under alkaline conditions, and then acidify it with sulfuric acid to obtain the product p-methoxybenzoic acid.
2 Research on the synthesis of p-methoxybenzoic acid in microchannel reactor
The synthesis of p-methoxybenzoic acid in a microchannel reactor uses a method using p-hydroxybenzoic acid as raw material. First, dissolve p-hydroxybenzoic acid into 30% alkali solution as one feed, and dimethyl sulfate as another feed. Convert the molar ratio of the materials into the volume ratio of the materials and then use an advection piston pump according to the Different flow ratios are introduced into the micro-channel reactor made of 316L stainless steel. During the reaction process, a hot and cold integrated machine is used for efficient heating to maintain a constant temperature in the reactor. The sodium salt of the product p-methoxybenzoic acid is obtained, and then acidified and precipitated with concentrated sulfuric acid to obtain the finished product.
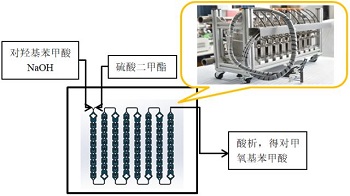
During the experiment, according to the reaction effect, the reaction material ratio, reaction temperature, and reaction residence time were adjusted in time to find the best reaction conditions.
3 Result analysis and discussion
3.1 Effect of reaction temperature on the synthesis of p-methoxybenzoic acid
The reaction ratio of the fixed materials is p-hydroxybenzoic acid: sodium hydroxide: dimethyl sulfate, which is 1:3.5:1.1. The residence time of the reaction is 60s. The effect of reaction temperature on the synthesis of p-methoxybenzoic acid is shown in the figure below.
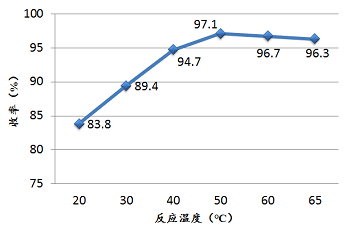
It can be seen from the figure that as the temperature increases, the yield of p-methoxybenzoic acid continues to increase. When the temperature reaches about 50°C, the yield reaches the maximum value. As the temperature increases, The raw materials are decomposed and the product impurities increase. Therefore, the optimal reaction temperature for this reaction in the microchannel reactor is basically calibrated to be about 50°C.
4 Summary
This article introduces the production process of synthesizing p-methoxybenzoic acid using a microchannel reactor independently developed and produced by Halma Chemical. Compared with the traditional kettle reaction, the yield of p-methoxybenzoic acid has increased from 93% to 93%. % has increased to more than 97%, and the optimal ingredient ratio of parahydroxybenzoic acid and dimethyl sulfate has dropped from about 1:1.2 to about 1:1.09. The reaction time has been shortened from the original 3-4 hours to the current 60 seconds. It is a very big improvement for the production process of p-methoxybenzoic acid.
References:
[1] Pennemann H, Watts P, Haswell S J, et al. Benchmarking of micro-reactor applications[ J]. OrgPro Res&Dev,2004,8:422 -439.
[2] P. Watts, S.J. Haswell, The application of micro reactors to organic synthesis. Chem. Commun., 391-8, 2001, issue 5.10644.
[3] P. Watts, S.J. Haswell, The application of micro reactors for organic synthesis. Chem. Soc. Rev., 34(3): 235-46, 2005.



 微信扫一扫打赏
微信扫一扫打赏
