Background and overview[1][2]
In the field of chemical pharmaceuticals, due to the introduction of fluorine atoms, most fluorine-containing organic compounds have specific properties that other organic compounds cannot match. The unique and excellent properties of fluorine-containing organic compounds have aroused great interest in them. In recent years, fluorine-containing intermediates have been studied by more and more scholars due to their excellent pharmacological and physiological activities. 1,3,5-Trifluorobenzene can be used as a pharmaceutical or liquid crystal material intermediate.
Apply[1-5]
1,3,5-Trifluorobenzene can be used as a pharmaceutical or liquid crystal material intermediate. Examples of its application are as follows:
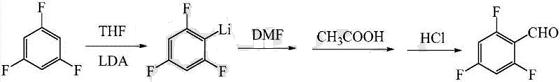
1. Preparation of 2,4,6-trifluorobenzaldehyde.
Fluorobenzaldehyde is an important organic chemical raw material and intermediate, which can be widely used in the synthesis of pesticides, medicines, dyes and resins. However, due to the relatively backward synthesis technology, there is a large gap between supply and demand. Therefore, developing new technologies for its preparation has good economic and social benefits. At present, the main synthesis methods reported for fluorobenzaldehyde include halogenated hydrolysis, direct chemical oxidation, electrochemical oxidation, oxygen oxidation, halogen exchange, fluorobenzoyl method, etc.
The preparation method includes: (1) preparing a tetrahydrofuran solution of lithium diisopropylamide and setting it aside for use; (2) cooling the temperature to -85~-80°C, and adding lithium diisopropylamide under nitrogen protection. The tetrahydrofuran solution is added dropwise to the tetrahydrofuran solution of 1,3,5-trifluorobenzene, and after the dropwise addition is completed, the reaction is kept warm for 3 to 5 hours; (3) Add dimethylformamide dropwise to the reaction solution, at -80~- Insulate at 75°C for 50-70 minutes; (4) Keep the temperature at -5-0°C, add glacial acetic acid, water and dilute hydrochloric acid to the reaction solution sequentially, adjust the pH to 1-2, and stir for 10-20 minutes after the addition is completed. A crude product is obtained; (5) The crude product is post-treated to obtain the target product 2,4,6-trifluorobenzaldehyde. The present invention uses non-nucleophilic organic base lithium diisopropylamide to provide a new idea for preparing 2,4,6-trifluorobenzaldehyde.
2. Preparation of 2,4,6-trifluorobenzylamine compound.
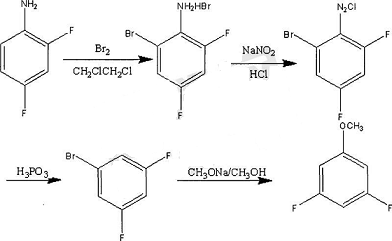
2,4,6-Trifluorobenzylamine is an important pharmaceutical and pesticide intermediate. One of its uses is the synthesis of the anti-AIDS drug Bictegravir. There are currently few reports on the synthesis of this compound. The main synthesis routes are: using 1,3,5-trifluorobenzene as raw material, n-butyllithium aldehyde, reduction with potassium borohydride, chlorination with thionyl chloride, and trifluorobenzene. A series of reactions including alkylation of lotropine into salts and hydrolysis yield 2,4,6-trifluorobenzylamine. This process route is cumbersome and difficult to operate, which is not conducive to industrial production.
Using pentachlorobenzonitrile as raw material, react with anhydrous potassium fluoride to obtain 3,5-dichloro-2,4,6-trifluorobenzonitrile; 3,5-dichloro-2,4,6- Add an organic base and a first catalyst to trifluorobenzonitrile, add hydrogen, and obtain 2,4,6-trifluorobenzonitrile through dechlorination and hydrogenolysis; add an acid and a second catalyst to 2,4,6-trifluorobenzonitrile. Catalyst, hydrogen gas is introduced, and 2,4,6-trifluorobenzylamine is obtained through cyano reduction. The total yield of the two steps of dechlorination, hydrogenolysis and cyano reduction is less than 60%. The literature also gives 3,5-trifluorobenzylamine. The one-step catalytic hydrogenation reduction of chloro-2,4,6-trifluorobenzonitrile gives 2,4,6-trifluorobenzylamine, but the yield is less than 30%.
Some research has developed a new route, using 1,3,5-trifluorobenzene as raw material, to prepare 2,4,6-trifluorobenzyl through lithiation, aldehyde, reduction, halogenation and displacement reactions. The amine method has mild reaction conditions, strong operability, high atom economy, simple process and easy industrialization, and the product has high purity and stable quality, meeting the requirements for use as an intermediate.
3. Preparation of 3,5-difluoroanisole.
3,5 difluoroanisole is an important fine organic synthesis intermediate, mainly used in the synthesis of pesticides, medicines and liquid crystal materials. By reacting 1,3,5-trifluorobenzene with sodium methoxide solution in the presence of a solvent, a reaction product is obtained, and then the reaction product is post-processed to obtain 3,5-difluoroanisole, The prepared product 3,5-difluoroanisole has high purity, high product yield, few reaction steps, and short product production cycle.
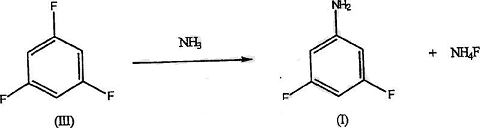
4. Preparation of 3,5-difluoroaniline.
The compound 3,5-difluoroaniline is a key intermediate in the synthesis of many broadleaf herbicides and other agricultural chemicals and pharmaceutical products. Many routes to the synthesis of this compound have been investigated. However, many such syntheses are difficult, do not provide adequate yields, or are simple but expensive. For example, it is difficult to introduce two fluorine substituents into positions 3 and 5 relative to amino or nitro functionality. To this end, technically complex and expensive synthesis schemes have been attempted.
However, unfavorable yields and large amounts of interfering reaction by-products make this type of synthesis impractical. In the field of agrochemicals, cost studies have repeatedly shown that only short synthesis routes (3 steps or less) are economically feasible. It has been studied that 3,5-difluoroaniline can be synthesized in high yield and purity by the following method: fluorination of 1,3,5-trichlorobenzene in the presence of a polar solvent to prepare the intermediate 1,3,5-trichlorobenzene. Fluorobenzene, and then amination of 1,3,5-trifluorobenzene in the presence of ammonia or anhydrous ammonia to obtain the desired 3,5-difluoroaniline. The details are as follows: A 600 ml Hastelloy pressurized reactor (Parr Instrument Co., Moline, IL) equipped with a standard stirrer, thermowell and valve was used.
Add 0.833 moles of 1,3,5-trifluorobenzene (110g), 4.6 moles of 29% ammonia water (270g) and 0.44 moles of magnesium oxide (17.8g) into the reactor. The reactor was flushed with argon and moderately heated to 225°C until maximum pressure was reached (approximately 1400 psig). As the reaction proceeds, the pressure is reduced at a rate of about 20 lbs./hour for about 6 hours. The reactor was then cooled and the contents allowed to settle. Drain the oil from the bottom of the reactor until salt/water is observed. The salt floats on the oil and slowly settles in the ammonia. The aqueous salt is extracted 2 or 3 times with MTBE.
The MTBE was combined with the oil and added to a distillation column equipped with stainless steel packing (ProPak). Centrifuge to separate the salts, wash with water and discard. The product was isolated by distillation of MTBE at atmospheric pressure and then DFA at 20-50 mm. 1,3,5-Trifluorobenzene in DEG First, a certain amount of 1,3,5-trifluorobenzene (TFB) and anhydrous NH3 were reacted in diethylene glycol (DEG) at 200°C. The reaction was unacceptably slow and the conversion to product was approximately 17% in 3 hours. The reaction was then carried out at 230°C. Maximum pressure is 960psig. After 10 hours, the conversion rate of TFB to 3,5-difluoroaniline (DFA) was 95%. The total accountability of gas chromatography analysis was 97%.
Preparation [5]
The raw material compound 1,3,5-trichlorobenzene (II) is treated with potassium fluoride in a solvent. A small amount of by-products including 3,5-difluorochlorobenzene (IV) and a large amount of potassium chloride are produced, together with the desired intermediate compound 1,3,5-trifluorobenzene (III).
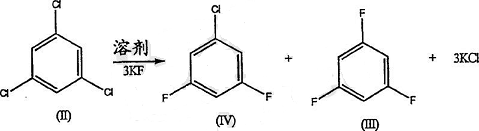
The specific steps are as follows: A 2-gallon (7.8L) stirred stainless steel pressurized reactor is installed into a 1″×10″ stainless steel distillation column filled with ProPak extruded metal packing. The top of the tower is equipped with a reflux condenser and a needle valve for discharging gas and taking out products. Install a thermocouple directly above the tower packing. The tower is wrapped with heating tape and insulated to minimize heat loss. To the reactor were added 33.4 moles of anhydrous N,N’-dimethylimidazolidinone (DMI) (3800g), 6.15 moles of 1,3,5-trifluorobenzene (TCB) (1118g) and 22.2 moles of fluorinated Potassium (KF) (1286g). The reactor is then sealed. Charge with nitrogen twice (200 psig) and then vent to remove air.
The reactor was then stirred vigorously (760 r.p.m.) and heated to about 310°C to about 315°C. After reaching 310°C, the nitrogen was evacuated from the reactor through the valve at the top of the condenser. After heating for about 2 to about 2.5 hours, the produced TFB is slowly evaporated through the outlet valve at the top of the condenser at a rate of about 100 ml/hr. TFB is removed in about 6 to about 7 hours. The reactor was then cooled and the salts were filtered from the DMI. Wash the salt with dichloromethane to recover all DMI. Other solvents such as methyl tert-butyl ether (MTBE) can also be used. The filtrate and solvent washes are combined and distilled to recover both the wash solvent and DMI. MTBE is distilled at atmospheric pressure, while DMI is recovered by vacuum distillation at about 118°C and 28 mmHg. To achieve high purity, TFB must be distilled again.
Main reference materials
[1] CN201610788956.1 A preparation method of 2,4,6-trifluorobenzaldehyde
[2] CN201410853129.7 Preparation method of 2,4,6-trifluorobenzylamine compound
[3] Preparation method of CN200810084162.23,5-difluoroanisole
[4] CN201810561854.5 A synthesis method of 2,4,6-trifluorobenzylamine
[5] CN01804645.2 Method for preparing 3,5-difluoroaniline from 1,3,5-trichlorobenzene
font-variant:normal; font-weight:400; letter-spacing:normal; list-style-image:none; list-style-position:outside; list-style-type:none; margin-bottom:0px; margin- left:0px; margin-right:0px; margin-top:0px; orphans:2; padding-bottom:0px; padding-left:0px; padding-right:0px; padding-top:0px; text-align:left; text-decoration:none; text-indent:0px; text-transform:none; vertical-align:middle; white-space:normal; word-spacing:0px” />
The specific steps are as follows: A 2-gallon (7.8L) stirred stainless steel pressurized reactor is installed into a 1″×10″ stainless steel distillation column filled with ProPak extruded metal packing. The top of the tower is equipped with a reflux condenser and a needle valve for discharging gas and taking out products. Install a thermocouple directly above the tower packing. The tower is wrapped with heating tape and insulated to minimize heat loss. To the reactor were added 33.4 moles of anhydrous N,N’-dimethylimidazolidinone (DMI) (3800g), 6.15 moles of 1,3,5-trifluorobenzene (TCB) (1118g) and 22.2 moles of fluorinated Potassium (KF) (1286g). The reactor is then sealed. Charge with nitrogen twice (200 psig) and then vent to remove air.
The reactor was then stirred vigorously (760 r.p.m.) and heated to about 310°C to about 315°C. After reaching 310°C, the nitrogen was evacuated from the reactor through the valve at the top of the condenser. After heating for about 2 to about 2.5 hours, the produced TFB is slowly evaporated through the outlet valve at the top of the condenser at a rate of about 100 ml/hr. TFB is removed in about 6 to about 7 hours. The reactor was then cooled and the salts were filtered from the DMI. Wash the salt with dichloromethane to recover all DMI. Other solvents such as methyl tert-butyl ether (MTBE) can also be used. The filtrate and solvent washes are combined and distilled to recover both the wash solvent and DMI. MTBE is distilled at atmospheric pressure, while DMI is recovered by vacuum distillation at about 118°C and 28 mmHg. To achieve high purity, TFB must be distilled again.
Main reference materials
[1] CN201610788956.1 A preparation method of 2,4,6-trifluorobenzaldehyde
[2] CN201410853129.7 Preparation method of 2,4,6-trifluorobenzylamine compound
[3] Preparation method of CN200810084162.23,5-difluoroanisole
[4] CN201810561854.5 A synthesis method of 2,4,6-trifluorobenzylamine
[5] CN01804645.2 Method for preparing 3,5-difluoroaniline from 1,3,5-trichlorobenzene



 微信扫一扫打赏
微信扫一扫打赏
