Application【1】
O-Methoxyaniline is used in the dye industry as a dye intermediate: used as a corrosion inhibitor for steel preservation; used as an antioxidant in some resins; in some European countries, used in the manufacture of pharmaceutical products and chemical textiles.
Exposure route【2】
Workers involved in the production of azo dyes may be poisoned by exposure to this substance; o-methoxyaniline is also found in cigarette smoke, so smoking may cause poisoning; in emissions from chemical plants and petroleum refineries Found to contain o-methoxyaniline.
Toxicological information【2】
It is highly toxic. The o-methoxyaniline that enters the body directly (or indirectly) oxidizes hemoglobin into a large amount of methemoglobin, forming methemoglobinemia, leading to tissue hypoxia and varying degrees of cyanosis. The intermediate products of this compound in the body can reduce reduced glutathione and increase the fragility of red blood cell membranes. Some of them can also directly combine with the sulfhydryl groups of globin in red blood cells to denature the globin to form globin inclusion bodies (i.e. denatured globin). corpuscles), eventually leading to erythrocyte rupture and hemolysis. The compound or metabolite may also directly (or indirectly) cause kidney damage.
Acute poisoning:
Rat—LD50 (oral): 1 400 mg/kg.
Mouse—LD50 (1:3 dose): 422 mg/kg.
Rabbit—LD50 (oral): 870 mg/kg.
Reproductive toxicity: Intraperitoneal test on rats, rabbits and other animals, moderate toxicity.
Mutagenicity: DNA inhibition (oral administration to mice): 200 mg/kg; microsomal mutagenicity (Salmonella typhimurium): 333 mol/L; DNA damage (abdominal administration to mice): 1 2509 mol/L.
Carcinogenicity: NCI (National Cancer Institute) reports that oral tests show that male rats can cause kidney and thyroid cancer, and both male and female rats have been found to suffer from bladder cancer.
Clinical symptoms【2】
Acute occupational poisoning is caused by inhaling a large amount of relevant poisons or absorbing them through the skin in a short period of time. It usually occurs during working hours or a few hours after get off work. Mild poisoning: Mild cyanosis of lips, auricles, tongue and fingernails, which may be accompanied by dizziness, headache, fatigue, and chest tightness. Methemoglobin is 10% to 30%, and usually returns to normal within 24 hours. Moderate poisoning: The skin and mucous membranes are obviously cyanotic, and symptoms such as palpitations, shortness of breath, loss of appetite, nausea, and vomiting may occur. Methemoglobin is between 30% and 50%; or mild hemolytic anemia, Hern’s bodies (i.e. beads) may occur. Protein bodies) can be higher than 20%; or chemical cystitis. Severe poisoning: severe cyanosis, lead-grey skin and mucous membranes, disturbance of consciousness, methemoglobin higher than 50%; or severe hemolytic anemia, Hern’s bodies may be higher than 50%; or severe liver and kidney damage .
International organizations and countries control indicators IARC【2】
It belongs to Group 2B carcinogens. There is insufficient evidence of carcinogenicity to humans and sufficient evidence of carcinogenicity to animals.
United States
NIOSH: The recommended limit concentration for exposure to air containing o-methoxyaniline is 0.5 mg/m3. The concentration that can immediately pose a threat to life and health is 50 mg/m3.
ACGIH: The time-weighted average concentration of o-methoxyaniline in the air should not exceed 0.1 ppm, and pointed out that absorption through the skin is the main route of o-methoxyaniline poisoning.
OSHA: Most workers can be exposed to it, and the maximum allowable concentration without serious effects on health is 0.5 mg/m3 when working 8 hours a day (40 hours a week)
California regulations: The 1996 recommendation on carcinogens in California stated that the carcinogenic potential index of o-methoxyaniline is 4.4×10-5kg/m3. In other words, a person who has been exposed to o-methoxyaniline at a concentration of 1 μg/m throughout his life has a cancer risk of no more than 40 per million.
Belgium, Australia, Germany, etc.
The time-weighted concentration threshold for long-term exposure of workers is 0.1 ppm (0.5 mg/m3), and the maximum concentration threshold for short-term exposure is 1 ppm. And pointed out that absorption through the skin is o-methoxyaniline. The main route of poisoning.
UK
It is stipulated that the concentration threshold for workers to be in contact with the substance for 8 hours is 0.5 mg/m3, the concentration threshold for 8 to 10 hours of contact with the substance is 0.35 mg/m3, and the concentration threshold for workers to be in contact with the substance for 12 to 16 hours is 0.5 mg/m3. The value is 0.125mg/m3, and the concentration threshold when in contact with the substance for more than 16 hours is 0.05mg/m3. It is pointed out that absorption through the skin is the main route of o-methoxyaniline poisoning. Suspected carcinogen in humans.
Switzerland
The time-weighted concentration threshold that stipulates that workers can be exposed to o-methoxyaniline for a long time is 0.1. ppm (0.5 mg/m3), the minimum value of the maximum concentration for short-term exposure is 0.2ppm (1mg/m3), and it is pointed out that absorption through the skin is the main way of poisoning this substance, and the longest time that you can be in contact with this substance 4 hours per day (for one month); one
China’s existing control indicators
GB/T 18885–2002 Technical requirements for ecological textiles: dyes that can decompose o-methoxyaniline under reducing conditions are prohibited dyes.
GB 4287-92 Textile Dyeing and Finishing Industry Water Pollutant Emission Standard: The maximum allowable emission concentration level one standard and level two standard for textile dyeing and finishing industry construction projects established on July 1, 1992 and for enterprises that are put into operation after completion. The standard and third-level standards are both 0.5 mg/L.
GB 14374–93 Aerospace propellant water pollutant emission standards: construction projects established before December 1, 1993The maximum allowable emission concentration of o-methoxyaniline for the project and the enterprises that will be put into production after its completion is 3.0 mg/L. The maximum allowable emission concentration of o-methoxyaniline for the construction project that was established on December 1, 1993 and the enterprises after its completion The concentration is 2.0
GB 16297–1996 Comprehensive Emission Standard of Air Pollutants: Established on January 1, 1997 (including new construction, expansion, and reconstruction)
The maximum concentration of o-methoxyaniline emitted by the pollution source through the exhaust pipe shall not exceed 20 mg/m3. The maximum allowable emission rate is stipulated according to the height of the exhaust pipe. An exhaust pipe with a height of 15m will emit o-methoxyaniline in any 1 hour. The rate of aniline is prohibited in the first-level standard, the second-level standard shall not exceed 0.52 kg/h, the third-level standard shall not exceed 0.78 kg/h, and the maximum concentration limit of irregular emissions without passing through the exhaust pipe is 0 .40mg/m3.
GB 8978–1996 Comprehensive Wastewater Discharge Standard: The maximum allowable discharge concentration for units constructed before December 31, 1997 is 1.0 mg/L for the first level, 2.0 mg/L for the second level, and 2.0 mgm for the third level. The grade standard is 5.0 mgm.
Preparation method【3】
Industrial production is generally carried out as follows
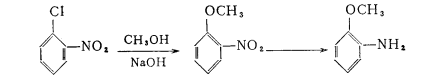
Boil 2 tons of water, 1.5 tons of iron powder and 15 kilograms of formic acid, slowly add 100 kilograms of nitrobenzene, then add 15 kilograms of formic acid and 1.5 tons of iron powder, and then add nitrobenzene 3 tons of methyl ether and 1 ton of iron powder. When the reduction is completed, let it stand, and then extract the supernatant liquid and use a small fractionating column for vacuum distillation. The iron residue is distilled with steam to extract the remaining methoxyaniline. Got 3,100 kg.
Production status【3】
Japan’s recent production capacity is 500-700 tons/year. Japan’s Junyang Pharmaceutical Company and Daiwa Chemical Company are major Japanese manufacturers and major exporters. Sumitomo Chemical’s production is mainly for its own use.
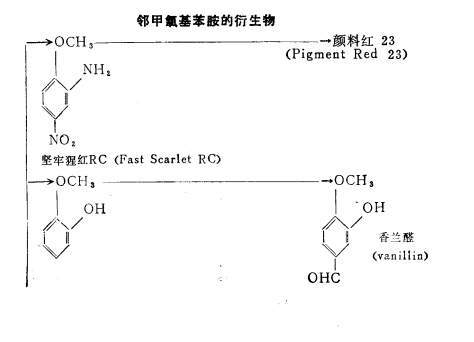
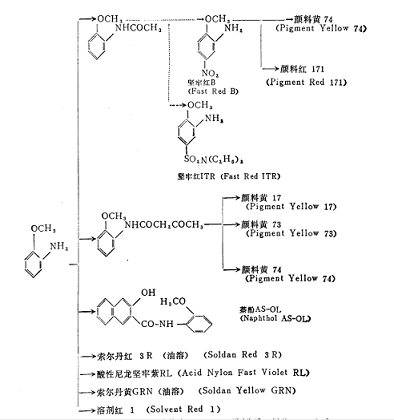
Purpose
The uses of o-methoxyaniline are shown in the figure below. For dyes and pigments, the estimated demand for dyes is 50-60%, and 40-50% for pigments. The demand for these dyes and pigments in Japan is relatively stable, so it is not expected that there will be significant growth in the future.
References
[1] Wang Dao, edited by Cheng Shuiyuan, Practical Handbook of Environmentally Hazardous Chemicals, China Environmental Science Press, 2007.12, page 248
[2] Institute of Science and Technology Information, Ministry of Chemical Industry, World Fine Chemicals Handbook, Institute of Science and Technology Information, Ministry of Chemical Industry, 1st edition, December 1982, page 517



 微信扫一扫打赏
微信扫一扫打赏
