Background and overview[1]
Dodecylbenzene is a long-chain linear alkylbenzene. It is a colorless and transparent liquid at room temperature with an aromatic smell. It can undergo sulfonation reaction, acylation reaction and cleavage reaction. It is the main intermediate for the production of detergents. Linear alkyl benzene sodium sulfonate synthesized from linear alkyl benzene has excellent surface activity, decontamination ability and biodegradability.
Synthesis method[1]
Weigh a certain amount of ZrOCl2·8H2O and dissolve it in an appropriate amount of distilled water to prepare a ZrOCl2 solution. Stir continuously and add a certain amount of TiCl4 (n(Zr)/n(T)=1.5) was slowly dropped into the above ZrOCl2 solution, and 25~28% ammonia was used to adjust the solution to pH=10. The obtained precipitate was aged at room temperature for 24 hours, filtered and washed until there was no Cl –, dried at 110°C for 24 hours and then ground to a particle size of 0.10 mm. Then, according to the ratio of 16g precipitate/100mLH2SO4, immerse it in 1mol/L sulfuric acid solution for 0.5h, filter, wash, dry, and heat at 500℃ Calculate for 3 hours to prepare SO42-/ZrO2-TiO2 catalyst. Add 15mL benzene and 3mL 1-dodecene into a 100mL two-necked flask with magnetic stirring and reflux condenser, then add 0.36g SO42-/ZrO2-TiO2 catalyst, reflux for 3 hours with constant stirring. The influence of the catalyst regeneration method on the synthesis of dodecylbenzene in this reaction is as follows.

Application fields[2][3]
1. Preparation of plasticizer
Alkylsulfonyl chlorides are widely used in industry, such as used in the manufacture of sodium alkyl sulfonate, a synthetic detergent, phenyl alkyl sulfonate, a plasticizer for polyvinyl chloride resin, especially alkyl sulfonate. Resin (called T-50 petroleum ester in industry) has excellent electrical properties, weather resistance and low heat consumption, and has been widely used as a plasticizer in various polyvinyl chloride products, especially in wire and cable formulations. The method of synthesizing phenyl dodecylbenzene sulfonate from dodecylbenzene is as follows: 1200kg dodecylbenzene, specific gravity 0.871 (15℃), a reaction kettle made of vinyl chloride Inside, the kettle is equipped with lead coils for cooling, mercury arc lamps, gas distributors, thermometers, gas discharge pipes, etc., and chlorine and sulfur dioxide with a ratio of 1:1.1 are introduced into the kettle. After mixing, the chlorine and sulfur dioxide are introduced at room temperature and turned on at the same time. Light, wavelength 280nm, the ratio of light power to dodecylbenzene amount is 0.8 W/kg. The reaction is exothermic. Use cooling water to control the temperature at 25℃~32℃, and pass in the mixed gas The amount is 30kg/h. The reaction generates hydrogen chloride and a small amount of unreacted gas, which is sprayed with water through the exhaust pipe and called hydrochloric acid recovery. When the specific gravity of the reactants reaches 0.996, stop feeding the gas and blow away the dissolved residual gas with dry compressed air. Afterwards, 1440kg of dodecylbenzenesulfonyl chloride was obtained, with a saponification value of 163 and a conversion rate of 45%.
2. Preparation of surfactant
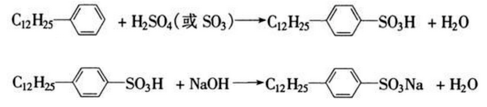
Dodecylbenzene can be used to synthesize dodecylbenzene sulfonate such as sodium dodecylbenzene sulfonate, which is a common anionic surfactant and is widely used in detergents, cleaning agents, etc. By adding 35 mL (34.6 g) of dodecylbenzene into a 250 mL four-neck bottle equipped with a stirrer, thermometer, dropping funnel and reflux condenser, slowly add 35 mL of 98% sulfuric acid under stirring, and the temperature does not exceed 40 ℃, after the addition is completed, the temperature is raised to 60 ~ 70 ℃, and the reaction is carried out for 2 hours. Cool the sulfonation mixture to 40~50℃, slowly add an appropriate amount of water (about 15mL) dropwise, pour it into a separatory funnel, let it stand for a moment, separate the layers, let go of the lower layer (water and inorganic salts), and keep the upper layer (organic phase). ). Prepare 80 mL of 10% mass fraction sodium hydroxide solution, add about 60 to 70 mL of it into a 250 mL four-neck bottle, slowly add the above organic phase dropwise while stirring, control the temperature to 40 to 50°C, and adjust with 10% mass fraction of sodium hydroxide. pH=7~8, and record the total dosage of 10% sodium hydroxide by mass fraction. In the above reaction system, add a small amount of sodium chloride, filter after the permeability circle test is clear, and obtain a white paste product, which is sodium dodecylbenzene sulfonate. The reaction formula is as follows.
Main references
[1] Wang Zhicai, Sun Kang, Liu Jiuling. SO42-/ZrO2-TiO2 catalyzed alkylation of benzene and 1-dodecene to synthesize dodecylbenzene[J]. Molecular Catalysis, 2007, 21(1): 38-42.
[2] Ren Zhi, Wang Zuming, Manufacturing method of alkylbenzene phenyl sulfonate series plasticizers, CN 92108526, application date 1992-08-08
[3] Basic Chemistry Experiment Writing Group of School of Chemical Engineering, Donghua University. Basic Chemistry Experiment. Shanghai: Donghua University Press, 2002



 微信扫一扫打赏
微信扫一扫打赏
