Background and overview[1][2]
The Chinese alias of 3-phenoxypropionic acid is 3-phenoxypropionic acid, CAS number 7170-38-9 2, chemical formula C9H10O3. Molecular weight 166.17400. Off-white crystalline powder, melting point 98-100 °C (lit.), boiling point 235-245 °C (lit.), flash point 243-245°C. 3-Phenoxypropionic acid can be used as an intermediate for pharmaceutical and chemical synthesis.
Apply[1]
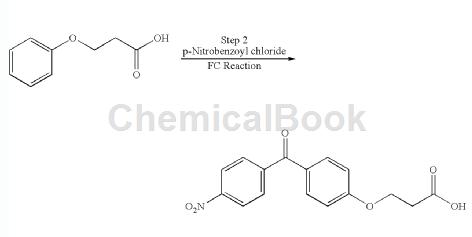
3-Phenoxypropionic acid can be used as an intermediate for pharmaceutical and chemical synthesis. For example, place 4-nitrobenzoic acid, thionyl chloride and DMF in a 3-liter 4-neck glass flask and reflux at 78°C. Heat for 2 hours. The completion of the reaction was monitored by the clarity of the reaction mixture.
Thionyl chloride was removed under reduced pressure. Dichloroethane (1 L) was added and evaporated to give 4-nitrobenzoyl chloride as a solid. The solid was dissolved in dichloroethane (1 L). Dichloroethane (10 L) was introduced into a 20 L glass flask and cooled to 0 °C. Add aluminum chloride at 0-5°C. Then, the 4-nitrobenzoyl chloride prepared above dissolved in dichloroethane was added dropwise at 0°C. Phenoxypropionic acid was added in portions to the 3-phenoxypropionic acid reaction composition at 0.5°C and stirred at 25°C overnight. Check TLC and quench the reaction composition in a mixture of hexanes (8 L) and ice-water (15 L). The composition was stirred and the solids were filtered off. The product was washed with hexane and dried. Yield: 400 g (70% of theory), Purity: 92.8% by HPLC.
Preparation [1-2]
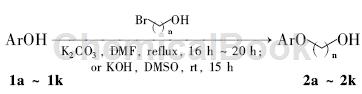
Method 1: Add 188 mg of phenol (2 mmol) and 10 ml of dry DMF into the reaction bottle, stir to dissolve; add 695 mg of 3-bromo-1-propanol (5 mmol) and 553 mg of anhydrous potassium carbonate , reflux the reaction to the end point (TLC detection). The reaction solution was diluted with ethyl acetate and filtered. The filtrate was washed with saturated brine, dried over anhydrous sodium sulfate, removed under reduced pressure and subjected to silica gel column chromatography [eluent: A = V (petroleum ether): V (ethyl acetate) Ester) = 3:1] purified to obtain 160 mg of 3-phenoxypropionic acid.
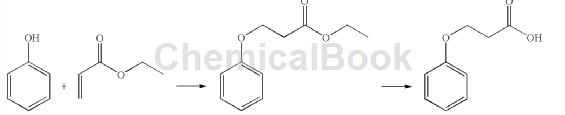
Method 2: Phenol and ethyl acrylate were introduced into a 5-liter three-neck glass flask equipped with a heating element and an overhead stirrer. Add Triton B and heat at reflux (120°C) for 48 hours. TLC showed that a small amount of phenol was still present. The reaction composition was processed as follows. The ethyl acrylate was removed and the residue was dissolved in ethyl acetate, washed with aqueous NaOH (10%), then with water (3.0 L) and dried over anhydrous sodium sulfate. The dry organic layer was concentrated to give a residue. The residue was placed in a 10.0 liter round-bottomed glass flask, concentrated HCl was added, and heated under reflux for 24 hours. Check TLC and cool the reaction composition to 25°C. The solid was filtered off and washed thoroughly with water (5 L). The product was vacuum dried in an oven at 30°C overnight. Yield: 450 g (27% of theory) Purity (HPLC): 98.32%
Main reference materials
[1] TRIN PHARMA GMBH Patent: US2010/234327 A1, 2010; Location in patent: Page/Page column 11; 15
[2] Zhang Zhijia, Li Jinhai, Chen Meijun, et al. Synthesis of new aryl-modified gambogic acid derivatives and their anti-tumor activity [J]. Synthetic Chemistry, 2015, 23(12): 1085-1094 .



 微信扫一扫打赏
微信扫一扫打赏
