Background and overview[1]
2-Bromomethyl-4-fluorobenzoic acid methyl ester Chinese aliases: N, N, N’, N’-tetramethyl-1,3-propanediamine, 1,3-bis(dimethylamino) Propane, density 1.534g/cm3, boiling point 291.6ºC at 760 mmHg), flash point 130.1ºC, refractive index 1.541, vapor pressure 0.00193mmHg at 25°C. 2-Bromomethyl-4-fluorobenzoic acid methyl ester can be used as a pharmaceutical and chemical synthesis intermediate.
Preparation [1]
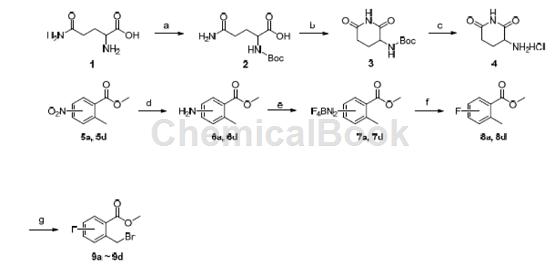
Step a: Synthesis of N-(tert-butoxycarbonyl)-L-glutamine (2)
14.6 g L-glutamine was dissolved in 100.0 mL 1.0 mol/L NaOH solution, and 21.8 g di-tert-butyl dicarbonate was slowly added dropwise in an ice bath. Remove the ice bath and stir at room temperature overnight. After the reaction, adjust the pH to 1 to 1.5 with potassium hydrogen sulfate, extract three times with 500 mL of ethyl acetate, combine the organic phases, dry with anhydrous sodium sulfate, and spin dry under reduced pressure to obtain 19.5 g of oil, yield 79 %. You can directly vote for the next step.
Step b: Synthesis of N-(2,6-piperidinedione-3-yl)carbamic acid tert-butyl ester (3)
19.0 g compound 2, 17.8 g EDCI and 12.6 g HOBt were dissolved in 200 mL acetonitrile, and 15.7 g triethylamine was slowly added dropwise at room temperature. The reaction solution was transferred to an oil bath and refluxed for 24 h. After the reaction, spin the solvent dry, add 500 mL of water, stir at room temperature for 1 hour, filter with suction, wash the filter cake with a large amount of water, rinse with a small amount of absolute ethanol, and drain to obtain 7.2 g of white solid with a yield of 41%. m.p. 214.4~214.5 ℃;
Step c: 3.2.3 Synthesis of 3-amino-2,6-piperidinedione hydrochloride (4)
Dissolve 1.4 g of N-(2,6-piperidinedione-3-yl)carbamic acid tert-butyl ester with 5.0 mL of 1,4-dioxane, and add 30 mL Stir 2.0 mol/L 1,4-dioxane hydrochloric acid solution at room temperature for 4 h. Filter, wash the filter cake with a small amount of ethyl acetate, and drain it to obtain 0.99 g of white solid, with a yield of 98%. m.p. 278.6~279.3 ℃;
Step d: Synthesis of 5d
Dissolve 9.0 g 2-methyl-3-nitrobenzoic acid in 40 mL anhydrous methanol, slowly add 15 mL SOCl2 dropwise, stir and react at room temperature for 0.5 h, and then transfer to an oil bath to reflux for 1 h. After the reaction, spin the solvent dry, add 200 mL of ethyl acetate, wash the organic phase once with saturated Na2CO3 solution and saturated saline solution, separate and dry; concentrate the organic phase and recrystallize to obtain 9.1 g of light yellow solid, yield 93 %. m.p. 65.5~66.1 ℃. You can directly invest in the next step of reaction.
Step e: Synthesis of methyl 2-amino-6-methylbenzoate (6d)
10.0 g compound 5d, 3.2 g ammonium chloride and 9.0 g zinc powder were placed in 200 mL ethanol/water (V/V=1/1) solution , 65 ℃ oil bath reaction. The reaction was monitored by TLC until the starting material was completely reacted. Rotate away most of the solvent, extract with 400 mL of ethyl acetate, dry the organic phase, concentrate, and use concentrated hydrochloric acid to form a salt. Filter with suction, wash the filter cake with ethyl acetate until white, and dry in vacuum to obtain 8.8 g of white solid with a yield of 85%.
Step f: Synthesis of methyl 2-fluoro-6-methylbenzoate (8d)
8.5 g of compound 6d was suspended in 32.4 g of 40% tetrafluoroboric acid and stirred for 10 min in an ice-salt bath. Dissolve 3.5 g of sodium nitrite in as little water as possible and slowly add it dropwise to the above mixed solution. Continue stirring for 1 h. Filter with suction and wash the filter cake with DCM until it turns light yellow. Suction dry to obtain 7.9 g of solid. m.p. 89.2~90.0 ℃.
Suspend 7.9 g of the above solid in 100 mL of toluene, slowly raise the temperature to 80 ℃, and then carefully raise the temperature to 85 ℃ after constant temperature. At this time, a large amount of white smoke will be produced. When the white smoke almost disappears, raise the temperature again and reflux 20 min. After the reaction is completed, cool to room temperature, add 100 mL Na2CO3 solution, and separate the liquids; dry the organic phase, concentrate, and separate by column chromatography [eluent: V (petroleum ether):V(ethyl acetate)=50:1] to obtain 2.5 g of colorless oil, with a yield of 24%.
Step g: Synthesis of 2-bromomethyl-6-fluorobenzoic acid methyl ester (9d)
550 mg compound 8a, 695 mg NBS and 40 mg BPO were dissolved in 30 mL CCl4 and refluxed in an oil bath for 6 h. After the reaction, spin the solvent dry, add 100 mL ethyl acetate, and add saturated NaHCO3 solution and Wash the organic phase once with saturated saline solution and separate the layers. The organic phase was dried, concentrated, and separated by column chromatography [(eluent: V (petroleum ether): V (ethyl acetate) = 50:1] to obtain compound 2 -Bromomethyl-4-fluorobenzoic acid methyl ester, colorless oilproduct, yield 23%.
Main reference materials
[1] Chen Yanchi, Ren Yujie. Synthesis of α-(isoindolinone-2-yl) glutarimide fluorinated analogs and their inhibitory activity on leukemia cells K562[J]. Organic Chemistry, 2015, 35 (5): 1123-1130.



 微信扫一扫打赏
微信扫一扫打赏
