Background and overview[1][2]
2,4-Difluorophenol can be used as an intermediate for pharmaceutical and chemical synthesis. The existing industrial production process of fluorinated phenol mainly adopts the method of diazotization and refluorination of p-aminophenol. When produced using this method, the product yield is low, a large amount of wastewater is generated, serious pollution and high cost; another The first production process is to use chlorinated nitrobenzene, undergo a fluorination reaction of chlorine atoms, then reduce the nitro group to an amine group, and the amine group is diazotized and hydrolyzed into fluorinated phenol. This method requires a multi-step reaction, and the total The yield is low and a lot of wastewater is produced during the process. It can be seen that the existing preparation methods of fluorinated phenol have problems such as high multi-step synthesis costs, cumbersome processes, waste of resources, and environmental pollution. In addition, there are reports in the literature about fluorination of phenol in solvents such as acetonitrile using fluorine gas as the fluorine source. Compared with traditional methods, this fluorination only requires one step to obtain the product, but the yield is lower. , the reaction products are complex, difficult to control, and the amount of solvent is large.
Apply[2]
2,4-Difluorophenol can be used as a pesticide, pharmaceutical and dye intermediate. Examples of its applications are as follows: Preparation of trichlorosine. “Tricloxin” is a broad-spectrum and highly effective antibacterial agent currently popular in the world. It has efficient and extensive killing and inhibiting effects on Gram-positive bacteria, negative bacteria, yeast and viruses, as well as antibiotic-resistant bacteria and non-antibiotic-resistant bacteria. It does not contaminate products and skin, has no irritating smell, and is safe for the human body and the environment. It is the world’s leading antibacterial agent, the best health care product and high-tech product. The process route using 2,4-dichlorophenol as the main raw material is as follows:
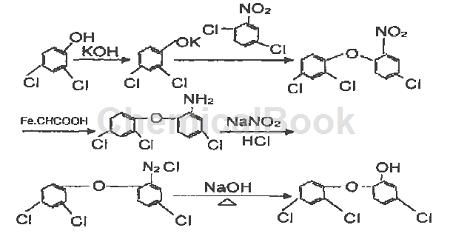
(1) Etherification reaction: In the etherification kettle, add a certain amount of distilled water, add potassium hydroxide under stirring, add 2,4-dichlorophenol after dissolution, and control the temperature to 100 ~120 ℃, react to produce potassium dichlorophenate, then add 2,5-dichloronitrobenzene, raise the temperature and operate under negative pressure, evaporate the water generated by the reaction and the original moisture, to The reaction ends at 160 ℃to produce 2,4,4′-2′-nitrodiphenyl ether. After alkali washing, crystallization and dehydration, the etherified product is obtained.
(2) Reduction reaction: Add a certain amount of water to the reduction kettle, add iron powder and glacial acetic acid under stirring, raise the temperature, and add a small amount of etherate evenly to perform the reduction reaction. The temperature was controlled to 100 ℃. After the reaction, sodium carbonate was added to adjust the pH value. Then add chlorobenzene for extraction, and filter the extracted product to remove iron sludge. The reduction product 2,4,4′-2′-aminodiphenyl ether can be obtained by recovering chlorobenzene through distillation.
(3) Diazotization reaction: In the diazotization kettle, add a quantitative mass fraction of 95% concentrated sulfuric acid, stir and control the temperature below 15 ℃, add sodium nitrite, and raise the temperature after adding About 60 ℃, add amino compounds to carry out diazotization reaction, and the product obtained is 2, 4, 4′-trichloro-2′-azodiphenyl ether.
(4) Hydrolysis reaction: Add a quantitative mass fraction of 75% sulfuric acid into the hydrolysis kettle, raise the temperature to 170 ℃, and flow diazotide for hydrolysis.
(5) Extraction: Taking advantage of the physical and chemical properties of the hydrolyzate, use toluene extraction. After removing the water, add alkaline water to dissolve the hydrolyzate in the alkaline water and separate toluene. Then the alkaline water is neutralized, so that the hydrolyzate is separated into an oil phase.
(6) Post-treatment: The crude trichloride product, the hydrolyzate, has been further increased in content through high vacuum distillation. Add it to an organic solvent, add activated carbon, etc. to decolorize and remove metal ions, and recrystallize to obtain a high-purity finished product. The reaction formula is as follows:
Preparation [1]
The preparation of fluorinated phenol by photocatalytic method solves the problems of low product yield, large amount of solvent required, high cost and environmental pollution in the current preparation process. Compared with other preparation methods, the present invention does not require other Solvent or catalyst, the raw materials are easy to be completely converted, the utilization rate of fluorine gas in the mixed gas is high, and the production cost is low. The process is as follows:
(1) Reaction: Add 30kg solid phenol into the reaction kettle, heat the reaction kettle with hot water to 40-50 ℃, so that the phenol is completely melted and stirred , then pass the fluorine-nitrogen mixture into the reaction kettle, and react for 5 hours under ultraviolet light irradiation.The temperature is 40-50℃; the flow rate of the fluorine-nitrogen mixture is 1.2L/s, and the volume fraction of fluorine in the fluorine-nitrogen mixture is 10%. After the reaction, blow with nitrogen Sweep for 30 minutes to obtain crude fluorinated phenol, and the purge gas and reaction tail gas enter the tail gas treatment process;
(2) Tail gas treatment: Send the purge gas and reaction tail gas to the activated carbon adsorption tower and solid soda lime absorption tower for adsorption in order to remove unreacted fluorine gas and acid gas in the reaction tail gas, and finally obtain Non-condensable gases are discharged to high altitude;
(3) Purification: The crude fluorinated phenol is separated by distillation to obtain 2-fluorophenol, 4-fluorophenol, 2,4-difluorophenol and 2,6-difluorophenol respectively.
Main reference materials
[1] CN201610231128.8 Process for preparing fluorinated phenol by photocatalytic method
[2] Production and application of trichlorine



 微信扫一扫打赏
微信扫一扫打赏
