Background and overview[1][2]
2’-Bromoacetophenone is an important organic synthesis intermediate and is widely used in spices, medicines, pesticides, dyes, organic optoelectronics and other fields. At present, the main preparation methods of 2’-bromoacetophenone are: Friedel-Crafts acylation method, 1-(2-bromophenyl)ethanol oxidation method and o-bromoethylbenzene oxidation method. Among them, most of the products of the Friedel-Crafts acylation method are para products, with very low yields and difficult to purify; the raw materials of the 1-(2-bromophenyl)ethanol oxidation method are relatively expensive and the steps are long, so the production conditions are not met. ; The oxidation method of o-bromoethylbenzene often uses high temperature, high pressure and expensive catalysts. The oxidation is not complete, making the raw material o-bromoethylbenzene difficult to remove and the purity is not high.
Apply[1-3]
2’-Bromoacetophenone is an important organic synthesis intermediate and is widely used in the synthesis of medicines, pesticides, dyes, flavors and fragrances, perfumes, etc. Examples of its application are as follows:
1. Preparation of 6,6,12,12-tetramethyl-6,12-dihydroindeno[1,2-b]fluorene
6,6,12,12-Tetramethyl-6,12-dihydroindeno[1,2-b]fluorene is an organic material. 6,6,12,12-Tetramethyl-6,12-dihydroindeno[1,2-b]fluorene can be prepared through bromination reaction to prepare 2,8-dibromo-6,6,12,12- Tetramethyl-6,12-dihydroindeno[1,2-b]fluorene, 2,8-dibromo-6,6,12,12-tetramethyl-6,12-dihydroindeno[1 , the two bromines of 2-b]fluorene are replaced by diarylamine compounds to prepare triarylamine compounds represented by formula (I), which can be used in organic electroluminescent device materials. The preparation method includes: coupling reaction: 2′-bromoacetophenone and 9,9-dimethylfluorene-2-boronic acid undergo a coupling reaction to generate the compound represented by formula M-1; wittig Reaction: The compound represented by formula M-1 and methyltriphenylphosphonium bromide undergo an addition wittig reaction to generate the compound represented by formula M-2; Cyclization reaction: The compound represented by formula M-2 is cyclized in the presence of acid reaction, it is converted into 6,6,12,12-tetramethyl-6,12-dihydroindeno[1,2-b]fluorene represented by formula M. The preparation method has a simple process and is easy to operate; the required raw materials are easy to obtain and low in cost; and the yield is as high as 66 to 78%, making it suitable for large-scale production.
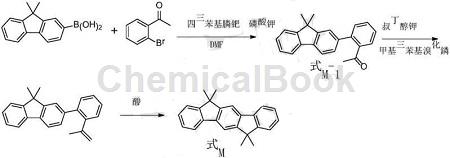
In addition, there is research to provide a preparation method for 6,6,12,12-tetramethyl-6,12-dihydroindeno[1,2-b]fluorene. The method includes: coupling reaction: 2′- bromoacetophenone and 9,9-dimethylfluorene-2-boronic acid are coupled to produce a compound represented by formula M-1; Formation reaction: the compound represented by formula M-1 undergoes an addition reaction with methylmagnesium bromide, and then hydrolyzes to generate the compound represented by formula M-2; cyclization reaction: the compound represented by formula M-2 is cyclized in the presence of acid reaction, it is converted into 6,6,12,12-tetramethyl-6,12-dihydroindeno[1,2-b]fluorene represented by formula M. The above preparation method has a simple process and is easy to operate; the required raw materials are easy to obtain and low in cost; and the yield is as high as 81~91%, making it suitable for large-scale production.
2. Preparation of spiro(1-tetralin-4,4’-piperidin-1-one) derivatives
Spiro(1-tetralin-4,4′-piperidin-1-one) is a relatively useful intermediate for drug synthesis. Currently, there is no practical method to synthesize spiro(1-tetralin-4). , 4′-piperidin-1-one) derivatives, therefore it is necessary to develop a simple and practical method to synthesize spiro (1-tetralin-4,4′-piperidin-1-one) derivatives. This avoids the shortcomings of current synthesis methods such as difficult access to raw materials, long routes, inconvenient reaction operations, difficult post-processing and low yields. Some studies have used the simple and easily available 2′-bromoacetophenone as raw material, reacted with 4-pyridinecarboxaldehyde in an alkaline solution to obtain the corresponding enone, which can be reduced by a reducing agent to obtain a saturated alcohol. , and then the saturated alcohol reacts with benzyl halide in an alkaline solution to obtain a quaternary ammonium salt, which is then reduced to obtain an alkene. The alkene is reduced and ring-closed by an alkyl tin reagent to obtain the corresponding benzyl-protected spiro(1,2,3,4-tetrahydrogen Naphthalene-4,4′-piperidine-1-hydroxy) derivatives. The products obtained by the present invention are mainly used as pharmaceutical intermediates.
Preparation [3-4]
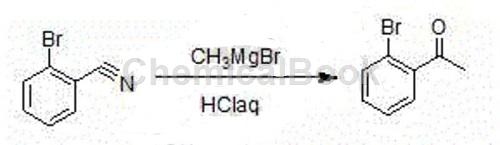
Method 1: The synthesis method of high-purity 2′-bromoacetophenone includes the following steps: add methylmagnesium bromide Grignard reagent in a container, and add o-bromine dropwise at room temperature. After adding the solution of benzonitrile in tetrahydrofuran, a large amount of white solid will appear; when the HPLC spectrum detects that there is no raw material, stop the reaction; add it dropwise to the hydrochloric acid solution, separate the liquid, spin dry, and distill under reduced pressure to obtain a colorless liquid product (High purity2′-bromoacetophenone product). The molar ratio of o-bromoxynil and methylmagnesium bromide is 1:1.05. The detailed steps are as follows: The input amounts are as follows: o-bromoxynil: 91g, methylmagnesium bromide (2M): 262.5ml, THF (Tetrahydrofuran, tetrahydrofuran): 90ml, concentrated hydrochloric acid: 60ml, toluene: 300ml. Add 262.5ml of methylmagnesium bromide (2M) into the 500ml reaction bottle, control the reaction temperature at 20-25℃, add dropwise 91g of o-bromoxynil and 90ml of THF solution, and control the reaction temperature at 20-25℃It takes about 2 hours to complete the dropwise addition. After the addition, a large amount of white solid will appear. React at this temperature for another 1 hour. Use HPLC to monitor the reaction. When the reaction liquid raw materials disappear, stop the reaction. Add the reaction liquid dropwise to 60ml concentrated hydrochloric acid and 300ml water, separate the liquids, extract the aqueous phase with 300ml toluene, combine the organic phases, wash with 200ml water, and reduce the pressure. -0.095MPa Concentrate until no more liquid distills out. Distill under reduced pressure, collect 131-135℃ (-0.095MPa) fraction, and obtain 95g of colorless transparent liquid 2’-bromoacetophenone. Determined density: 1.48 (20℃), reaction yield (based on o-bromoxynil) 95.5%.
Method 2: A method for preparing 2′-bromoacetophenone by bionic catalytic oxygen oxidation of o-bromoethylbenzene. This method uses o-bromoethylbenzene as raw material, under normal pressure and without Under the solvent, use any one or two combinations of 1~30ppm mononuclear metalloporphyrin and μ-oxy-dinuclear metalloporphyrin as the catalyst, and introduce oxygen at a flow rate of 10~60mL/min. Initiate the reaction at 170℃, and then react at 80~120℃ for 6~16h to obtain 2′-bromoacetophenone. The method of the present invention adopts high-temperature rapid initiation and low-temperature reaction, which makes the reaction initiation time extremely short, greatly shortens the reaction time, improves the reaction efficiency, reduces energy consumption, reduces operating costs, and increases reaction safety.
Main reference materials
[1] CN201610332456.7 A preparation method of 6,6,12,12-tetramethyl-6,12-dihydroindeno[1,2-b]fluorene
[2] CN200810043049.X A method for synthesizing spiro (1-tetralin-4,4’-piperidine) derivatives
[3] CN201610330780.5 Preparation method of 6,6,12,12-tetramethyl-6,12-dihydroindeno[1,2-b]fluorene
[4] CN201410518187.4 Synthesis method of high-purity 2′-bromoacetophenone



 微信扫一扫打赏
微信扫一扫打赏
