Background and overview[1][2]
In recent years, due to the use of single pesticides and unscientific use of drugs, the frequency and dosage of sterilization drugs have increased. As a result, various pathogens have become resistant to pesticides, which has become a major problem in chemical control; resistance is a shortening of The primary reason is the life cycle of new compounds. To study new compounds, there are problems such as long R&D cycles and high investment costs. Therefore, it is very difficult to screen out an effective compound. At the same time, frequent pesticide application increases the burden on farmers and intensifies environmental pollution.
Fenpropimorph, also known as Fenpropimorph and Probendazim, has the chemical name (±) cis 4-[3-(4tert-butylphenyl)-2-methylpropyl]-2 , 6-dimethylmorpholine. The pure product is a colorless, aromatic oily liquid with a boiling point of 392°C (101.3kPa). It is a systemic morpholine fungicide and ergot with preventive and therapeutic effects. Sterol reduction inhibitor. It can prevent and control important seedling diseases on cereal crops, legumes and sugar beets caused by Cercospora betaine, Powdery mildew of the genus Gramineae, Rhizoma rye, Puccinia betaine and Puccinia genus.
The existing synthesis method of fenpropimorph is to condense p-tert-butylbenzaldehyde and propionaldehyde to obtain 2-methyl-3-(p-tert-butyl)phenyl acrolein, which is obtained by selective hydrogenation. 2-Methyl-3-(p-tert-butyl)phenylpropanal is obtained by reacting with 2,6-dimethylmorpholine. The key step of this method is hydrogenation. The hydrogenation catalyst reported in the relevant literature is Pd/C or nickel complex. However, from the actual reaction point of view, in addition to producing 2-methyl-3-(p-tert-butyl)phenyl acrolein during the hydrogenation process, it is also easy to generate more by-products 2-methyl-3-(p-tert-butyl). Butyl) phenyl propanol, so it needs to be separated by distillation, and the separation of the two is difficult.
Mechanism of action[3]
The mechanism of action of fenpropimorph is similar to that of dodecamorpholine and tridecamorpholine. The bactericidal activity of fenpropimorph against pathogens is achieved by inhibiting the biosynthesis of ergosterol in the bacteria. Its action site is Sterol isomerization:
 , the double bond reduction process is inhibited, or sterol epoxidase is inhibited. A comparison of the 3 main morpholine fungicides is as follows:
, the double bond reduction process is inhibited, or sterol epoxidase is inhibited. A comparison of the 3 main morpholine fungicides is as follows:

Apply[2][4]
Bufenpropion is an ergosterol biosynthesis inhibitor, acting on Δ14-reductase and isomerization Δ8-isomerase ~ Δ7 isomerase. It is sprayed on the leaves and conducted through the xylem. It is low-toxic and highly effective. Systemic broad-spectrum fungicide with protective and curative activity; used to control grain powdery mildew, leaf blight and rust; can also control brown spot, rust and powdery mildew in sugar beets, leaf spot and rust in beans and leeks , banana leaf spot, and sunflower stem canker.
There is research and development of a bactericidal composition containing fenpropimorph. The active ingredients include a first active ingredient fenfenmorph and a second active ingredient. The second active ingredient is epoxiconazole, prothioconazole, and pentoconazole. Any one of triacetamin, mesostrobin, oxystrobin, flufenacet or fluazimid; the dosage form of the fungicidal composition is any dosage form allowed in agriculture, including granules, emulsifiable concentrate,Water-dispersible granules, wettable powders, microemulsions, suspoemulsions or microcapsule suspensions? Suspensions. The bactericidal composition can expand the scope of prevention and treatment targets, reduce the dosage of pesticides, and delay the development of resistance.
In addition, there is research and development of a special anti-rot agent for the succulent plant Swallow Palm, which is prepared from the following raw materials in parts by weight: 5-10 parts of sodium pentachlorophenol, 5-10 parts of phoxim parts, 3-5 parts of triacontanol, 2-4 parts of methyl chloroformate, 2-6 parts of sodium thiocyanate, 1-3 parts of o-phenylenediamine, 1-3 parts of aluminum triethylphosphonate, 20 parts of ethanol -30 parts, propiconazole 3-5 parts, diconazole 3-7 parts, nitrilebenzole 3-8 parts, amidazole 4-6 parts, oxanazole 4-8 parts, prochloraz 6-10 parts , 6-12 parts of imazalil, 8-10 parts of triclobutanol, 8-15 parts of pyrimethanol, 10-12 parts of chlorfenac, 5-8 parts of trimorpholine, 3-5 parts of fenpropimorph parts, dimethomorph 4-6 parts, fluopicolin 3-5 parts, boscalid 2-4 parts. The above-mentioned special anti-rot agents can effectively eradicate the occurrence of succulent plant rot diseases caused by various reasons.
Preparation [1,5]
Method 1: A method for synthesizing butyl:benzomorphine, including the following steps:
1). Dissolve 2-methyl-3-(p-tert-butyl)phenyl acrolein in a solvent and perform catalytic hydrogenation to obtain 2-methyl-3-(p-tert-butyl)phenyl. Propanol; the catalyst dosage is 0.5% to 10% of the weight of 2-methyl-3-(p-tert-butyl)phenyl acrolein, and the catalyst is Raney nickel;
2) Preparing a supported catalyst: The metal salt solution and the carrier are made into a supported catalyst. The metal in the supported catalyst accounts for 0.5% to 20% of the total weight. The metal salts are copper salts, nickel salts and cobalt salts. At least one of;
3), 2-methyl-3-(p-tert-butyl)phenylpropanol is condensed with 2,6-dimethylmorpholine under the action of a supported catalyst to obtain fenpropimorpholine; 2- The material ratio of methyl-3-(p-tert-butyl)phenylpropanol to 2,6-dimethylmorpholine is 1:0.5~1:5, and the amount of supported catalyst is 2-methyl-3 – 1% to 10% by weight of (p-tert-butyl)phenyl propanol.
Method 2: A synthesis method of fenpropimorph: using p-tert-butyl-β-methylphenylpropanol as the starting material, esterification with methylsulfonyl chloride or chlorination with thionyl chloride During the chemical synthesis: after the intermediate, the intermediate is synthesized with 2,6-dimethylmorpholine to generate fenfenmorpholine. The chemical equation is as follows:
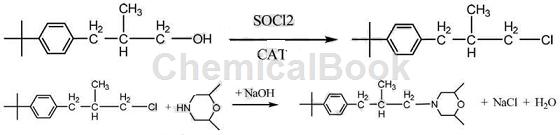
Includes the following steps:
(1) Esterification: Add methylsulfonyl chloride to p-tert-butyl-β-methylphenylpropanol, add triethylamine dropwise at 0-10°C, after the dropwise addition is completed, remove the freezer and let the temperature rise naturally Incubate for 1 hour; take samples for analysis until the GC normalized content of tert-butyl-β-methylphenylpropanol is ≤0.5% and the reaction is completed; then add water to dissolve the salt generated, let stand and separate into layers. The lower layer is the middle after the reaction. Body sulfonate;
(2) Condensation: Add 2,6-dimethylmorpholine to the sulfonate ester of the first step of esterification synthesis, raise the temperature and reflux until the temperature reaches 140°C, keep the temperature for 4 hours, take samples and analyze until the intermediate The GC normalized content of p-tert-butyl-β-methylphenylpropane chloride is ≤0.5%. The reaction is completed, and then neutralized with liquid caustic soda to PH=14. Leave to stand and separate into layers. The upper oil layer is distilled under negative pressure to remove the previous fraction. Finally, the finished product fenpropimorph is collected.
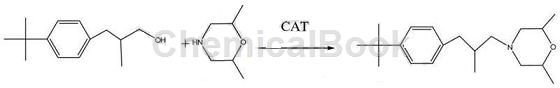
Method 3: Using tert-butylbenzene as the raw material, first undergo acylation to obtain 4 tert-butylpropiophenone through F-C reaction, and then perform Vilsmeier reaction with phosphorus oxychloride and alkali in dimethylformamide More than 95% of 4-tert-butylphenyl-1-chloro-2 methacrolein is obtained. The enal is hydrogenated and reduced to 4-tert-butylphenylisobutyraldehyde via Pd/C, and the saturated aldehyde is then mixed with 2 , 6-dimethylmorpholine reaction can produce fenpropimorph. The reaction equation is as follows:
Main reference materials
[1] CN201310218154.3 Synthesis method of fenpropimorph
[2] CN201810117739.9 A bactericidal composition containing fenpropimorph
[3] Development progress of fenpropimorph
[4] CN201510238428.4 Special anti-rot agent for the succulent plant Swallow Palm
[5] CN200610050100.0 A kind of synthesis method of fenpropimorph
13px; font-style:normal; font-variant:normal; font-weight:400; letter-spacing:normal; list-style-image:none; list-style-position:outside; list-style-type:none; margin-bottom:0px; margin-left:0px; margin-right:0px; margin-top:0px; orphans:2; padding-bottom:0px; padding-left:0px; padding-right:0px; padding-top: 0px; text-align:left; text-decoration:none; text-indent:0px; text-transform:none; vertical-align:middle; white-space:normal; word-spacing:0px” />
Main reference materials
[1] CN201310218154.3 Synthesis method of fenpropimorph
[2] CN201810117739.9 A bactericidal composition containing fenpropimorph
[3] Development progress of fenpropimorph
[4] CN201510238428.4 Special anti-rot agent for the succulent plant Swallow Palm
[5] CN200610050100.0 A kind of synthesis method of fenpropimorph



 微信扫一扫打赏
微信扫一扫打赏
